Erythromycin estolate tablet and preparation method thereof
A technology based on erythromycin and erythromycin tablets, applied in the direction of pharmaceutical formulations, medical preparations with no active ingredients, medical preparations containing active ingredients, etc., can solve the problem of affecting the uniformity and stability of tablet quality and affecting the quality of tablets , affecting the stability of drug efficacy and other issues, to achieve the effect of being suitable for industrial application, simple and easy to control the preparation method, and uniform and stable quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
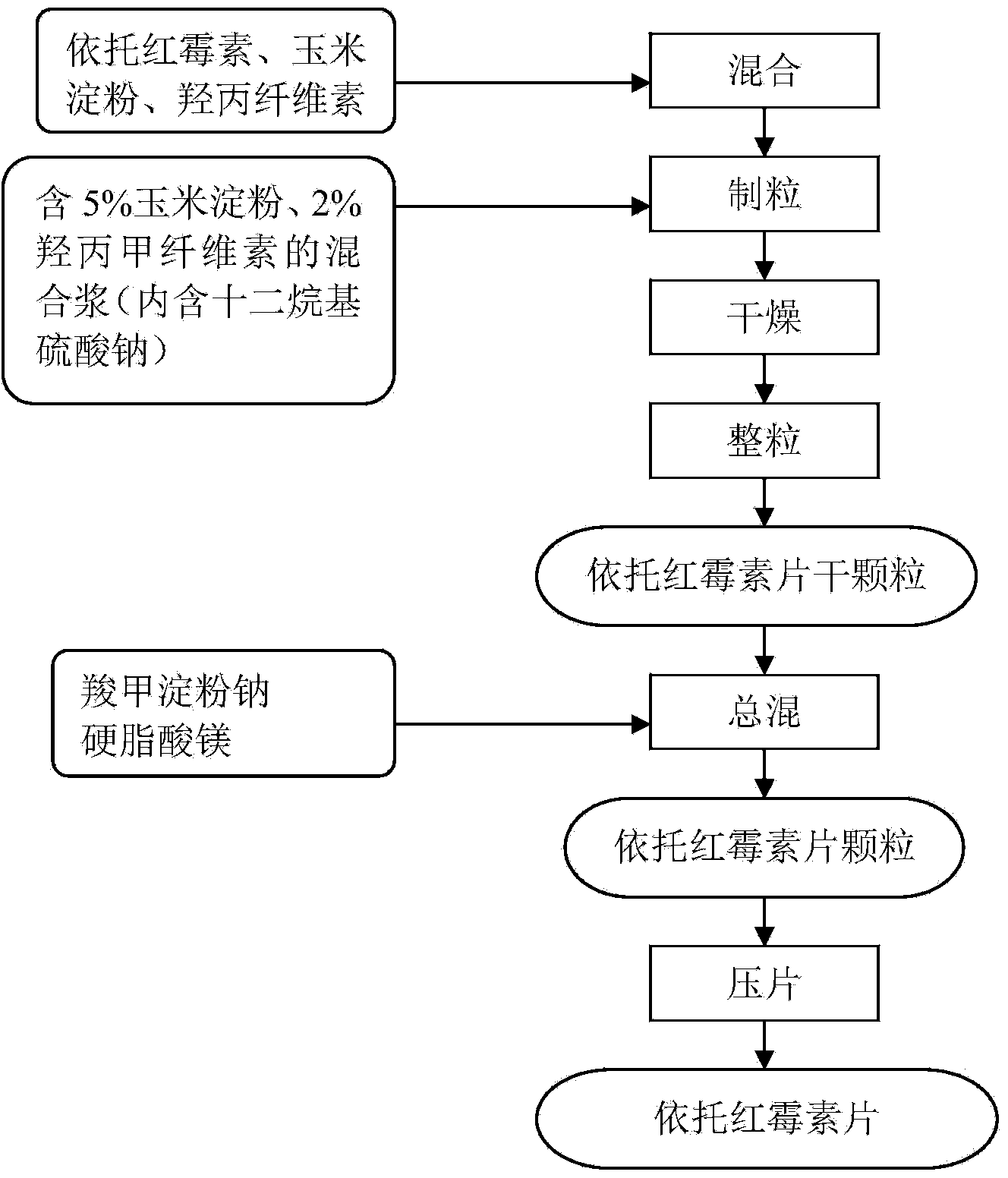
[0026] Etto erythromycin tablets are obtained through the preparation method of the following steps, and the schematic diagram of its preparation process is as follows: figure 1 Shown:
[0027] (1) Weighing raw materials by weight: 375 parts of erythromycin Eto, 79.5 parts of cornstarch, 30 parts of hypromellose, 5.4 parts of hypromellose, 1.44 parts of sodium lauryl sulfate, 249.6 parts of purified water , 7.38 parts of sodium starch glycolate, 5.88 parts of magnesium stearate;
[0028] (2) Preparation of granulation binder: In the pulping container, first dissolve 1.44 parts of sodium lauryl sulfate with a small amount of hot purified water, then add 24 parts of purified water and 13.5 parts of cornstarch to dilute into a suspension , weigh and add the remaining purified water heated to a temperature above 95°C, then add 5.4 parts of hypromellose and stir evenly quickly, punch the slurry, and stir evenly to obtain a mixed slurry, which is set aside;
[0029] (3) Wet granul...
Embodiment 2
[0034] Etto erythromycin tablets are obtained through the preparation method of the following steps, and the schematic diagram of its preparation process is as follows: figure 1 Shown:
[0035] (1) Weighing raw materials by weight: 375 parts of erythromycin Eto, 75 parts of cornstarch, 25 parts of hypromellose, 4 parts of hypromellose, 1 part of sodium lauryl sulfate, 240 parts of purified water , 6 parts of sodium starch glycolate, 4.5 parts of magnesium stearate;
[0036] (2) Preparation of granulation adhesive: first dissolve 1 part of sodium lauryl sulfate with a small amount of hot purified water, then add 23 parts of purified water and 12.7 parts of cornstarch, dilute into a suspension, weigh and heat to temperature Add the remaining purified water above 95°C, then add 4 parts of hypromellose and stir evenly, rinse the pulp, and stir evenly to obtain a mixed pulp, which is ready for use;
[0037] (3) Wet granulation: add 375 parts of Etoerythromycin and 25 parts of hyd...
Embodiment 3
[0042] Etto erythromycin tablets are obtained through the preparation method of the following steps, and the schematic diagram of its preparation process is as follows: figure 1 Shown:
[0043] (1) Weighing raw materials by weight: 375 parts of erythromycin Eto, 85 parts of cornstarch, 35 parts of hypromellose, 6 parts of hypromellose, 2 parts of sodium lauryl sulfate, 260 parts of purified water , 8 parts of sodium starch glycolate, 6 parts of magnesium stearate;
[0044] (2) Preparation of granulation adhesive: first dissolve 2 parts of sodium lauryl sulfate with a small amount of hot purified water, then add 25 parts of purified water and 14.4 parts of cornstarch, dilute into a suspension, weigh and heat to temperature Add the remaining purified water above 95°C, then add 6 parts of hypromellose and stir evenly, rinse the pulp, and stir evenly to obtain a mixed pulp, which is ready for use;
[0045](3) Wet granulation: Add 375 parts of Etoerythromycin and 35 parts of hydr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com