Method for producing clean fuel by adopting chlorine-containing plastic oil
A clean fuel and plastic oil technology, applied in the petroleum industry, processing hydrocarbon oil, preparation of liquid hydrocarbon mixtures, etc., to achieve the effects of inhibiting corrosion, improving selectivity, and prolonging life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
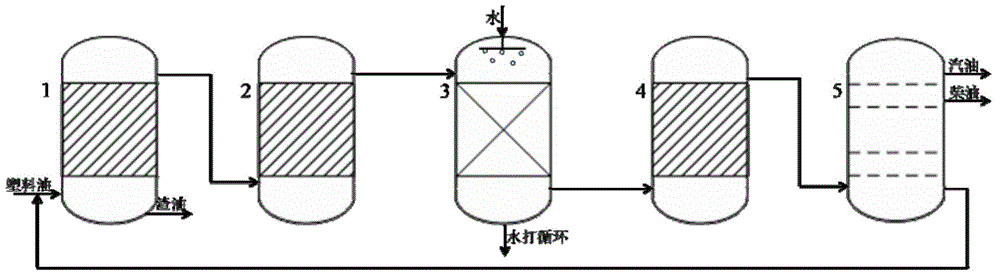
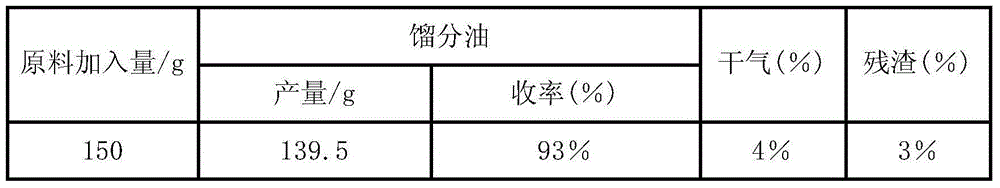
[0020] Gas-phase catalytic cracking of chlorinated plastic oils over molecular sieve / alumina catalysts. The chlorine-containing plastic oil is injected into a reactive distillation tower equipped with a molecular sieve / alumina catalyst for reaction and rectification. The catalyst is composed of alumina containing 35% ZSM-5 and 15% beta zeolite, obtained by bond molding 2.0-3.0mm columnar product, length 3-8mm, bulk density 0.75g / mL, strength greater than 40N / mm. The waste residue and gas generated during the waste plastic oil conversion process are used for heating, and the ratio of agent to oil is controlled at 15-20. See Table 1 below for the results of the catalytic distillation mass balance test.
[0021]
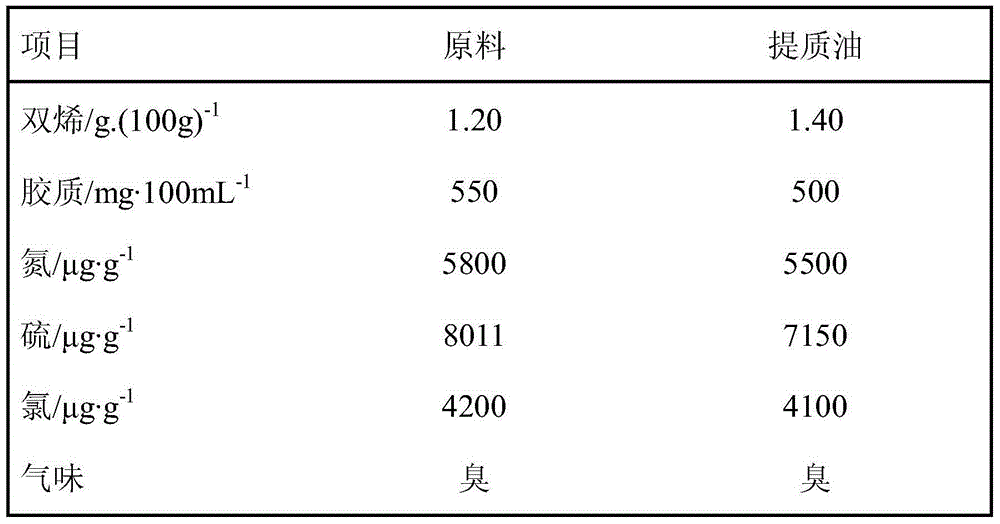
[0022] The following table 2 sees the properties of catalytic distillation chlorine-containing plastic oil
[0023]
[0024] It can be seen from Table 2 that after catalytic distillation of chlorine-containing plastic oil, the chlorine content basically does not...
Embodiment 2
[0026] The hydrodechlorination catalyst adopts supported Pd, Pt, Ru, Ir, Rh, Co or Ni metal catalysts. The carrier adopts SiO 2 -Al 2 o 3 , the specific surface area is 200-400m 2 / g, the pore volume is 0.5-2.0cm 3 / g, the most probable pore size distribution is 2-4nm and 10-15nm. The metal precursors are respectively palladium nitrate or palladium acetate, chloroplatinic acid, ruthenium trichloride, iridium trichloride, rhodium trichloride, cobalt nitrate and nickel nitrate or nickel acetylacetonate. The supported metal catalyst is prepared by an equal-volume impregnation method through the steps of impregnation-drying-calcination. For noble metals Pd, Pt, Ru, Ir and Rh, the metal loading is 3-10%; for metal Co and Ni, the metal loading is 30-60%. The following table 3 sees reaction process conditions and product properties.
[0027]
[0028]It can be seen from Table 3 that the dechlorination effect of all metal catalysts is obvious after low-pressure hydrodechlorin...
Embodiment 3
[0030] The effect of temperature and pressure on the effect of hydrodechlorination was investigated by taking high-nickel catalyst as an example. The metallic Ni metal loading was 55%. The following table 4 sees reaction process conditions and product properties.
[0031]
[0032] It can be seen from Table 4 that the reaction temperature has a greater influence on the dechlorination effect, while the reaction temperature has less influence on the hydrodechlorination effect.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Length | aaaaa | aaaaa |
| Bulk density | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com