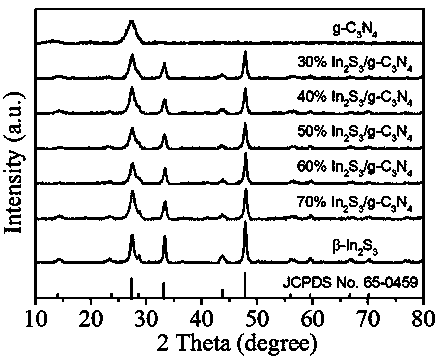
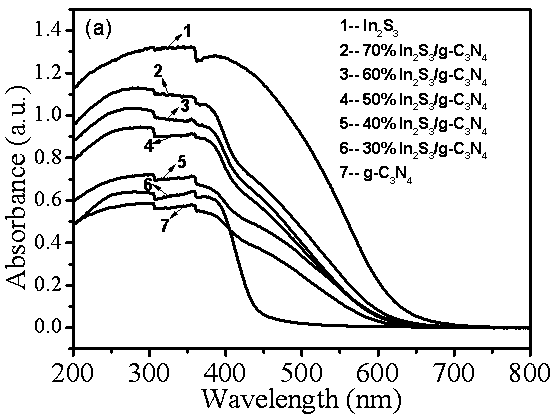
Preparation method of indium sulfide/carbon nitride composite nano material
A technology of composite nanomaterials and carbon nitride, applied in the field of photocatalytic materials, can solve the problems of expensive, unsuitable for large-scale production, and low quantum efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1 class graphitic nitrogen carbide (g-C 3 N 4 ) preparation
[0021] g-C 3 N 4 The preparation adopts the method of thermal polymerization of urea; weigh 10 g of urea in a semi-closed crucible and place it at 80 o C in a drying oven for 48 h, then transfer the crucible to a temperature-programmed tube furnace; set the temperature-programmed tube furnace at 2.3 o The heating rate of C / min is heated to 550 o C and keep it warm for 4 h; after cooling down to room temperature naturally, take it out, grind it into powder with a mortar, and use a concentration of 0.01 mol·L -1 dilute HNO 3 Wash 3 times to remove residual alkaline species, then wash 3 times with deionized water and absolute ethanol respectively, and finally wash at 80 o C oven dried for 12 h.
Embodiment 2
[0022] Example 2 30% In 2 S 3 / g -C 3 N 4 Preparation of composite materials
[0023] In 2 S 3 / g -C 3 N 4 Composite materials were prepared using the traditional hydrothermal method: weigh 0.2 g g-C 3 N 4 The powder was dissolved in 150 mL of pure water, then ultrasonicated for 0.5 h in an ultrasonic machine with a power of 250 W, and then In(NO 3 ) 3 4.5H 2 O 0.1406 g, stirred for 0.5 h, after completely dissolved, added dropwise to C 2 h 5 NS (0.05 mol L -1 ) solution 14.72 ml, and stirred for 0.5 h, then transferred to a 50 mL polytetrafluoroethylene-lined reactor, placed in an oven, at 160 ο C for 8 h, take it out and cool it down to room temperature naturally, wash the obtained sample 3 times with deionized water, wash 3 times with absolute ethanol, and place in a vacuum oven for 60 ο C was vacuum dried for 12 h to obtain 30% In 2 S 3 / g -C 3 N 4 composite material.
Embodiment 3
[0024] Example 3 40% In 2 S 3 / g -C 3 N 4 Preparation of composite materials
[0025] In 2 S 3 / g -C 3 N 4 Composite materials were prepared using the traditional hydrothermal method: weigh 0.2 g g-C 3 N 4 The powder was dissolved in 150 mL of pure water, then ultrasonicated for 0.5 h in an ultrasonic machine with a power of 250 W, and then In(NO 3 ) 3 4.5H 2 O 0.1875 g, stirred for 0.5 h, after completely dissolved, added dropwise to C 2 h 5 NS (0.05 mol L -1 ) solution 19.63 ml, stirred for another 0.5 h, then transferred to a 50 mL polytetrafluoroethylene-lined reactor, placed in an oven, at 120 ο C for 12 h, take it out and cool it to room temperature naturally, wash the obtained sample with deionized water for 3 times, wash with absolute ethanol for 3 times, and place in a vacuum oven for 60 ο C was vacuum dried for 12 h to obtain 40% In 2 S 3 / g -C 3 N 4 composite material.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com