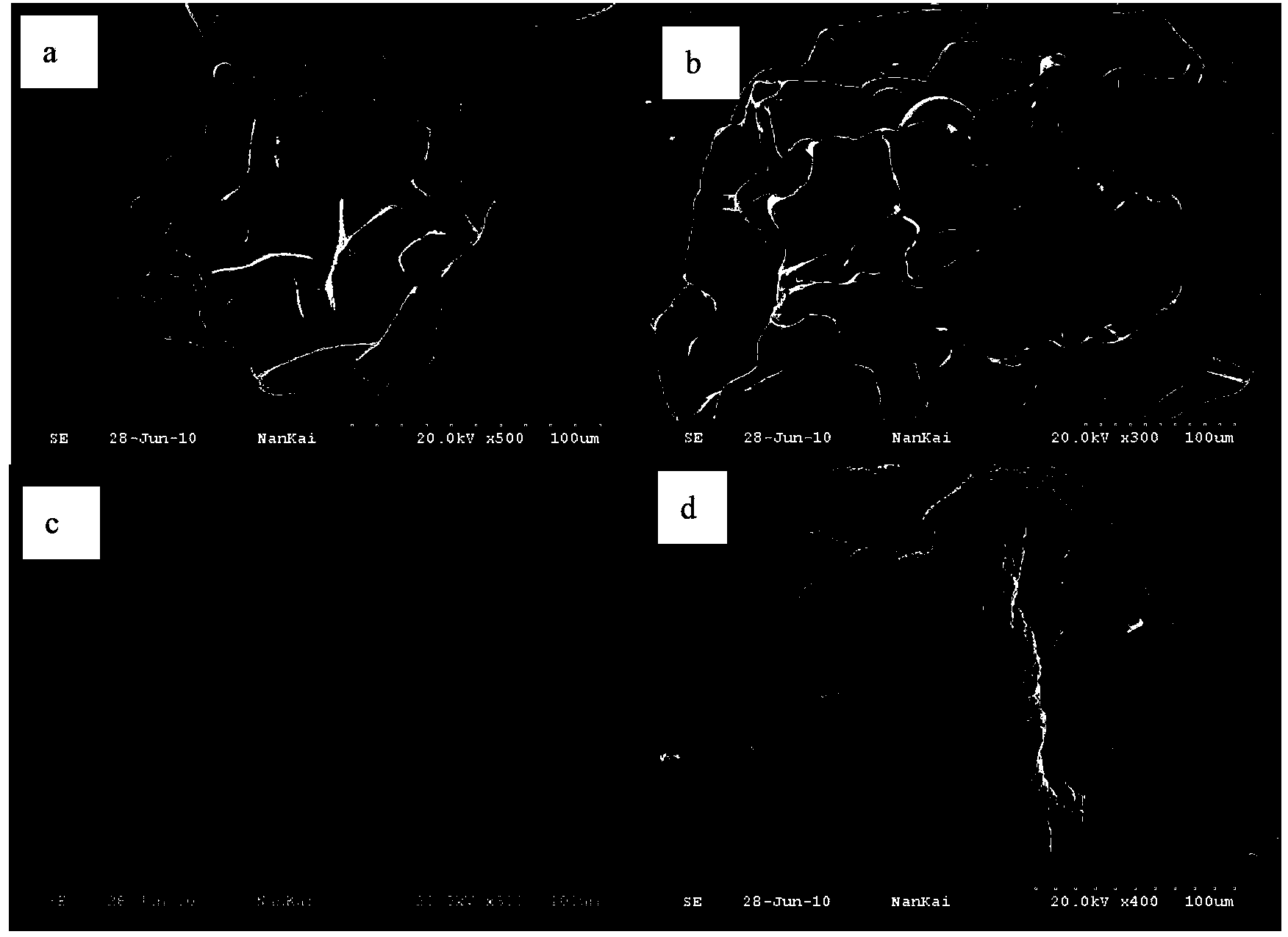
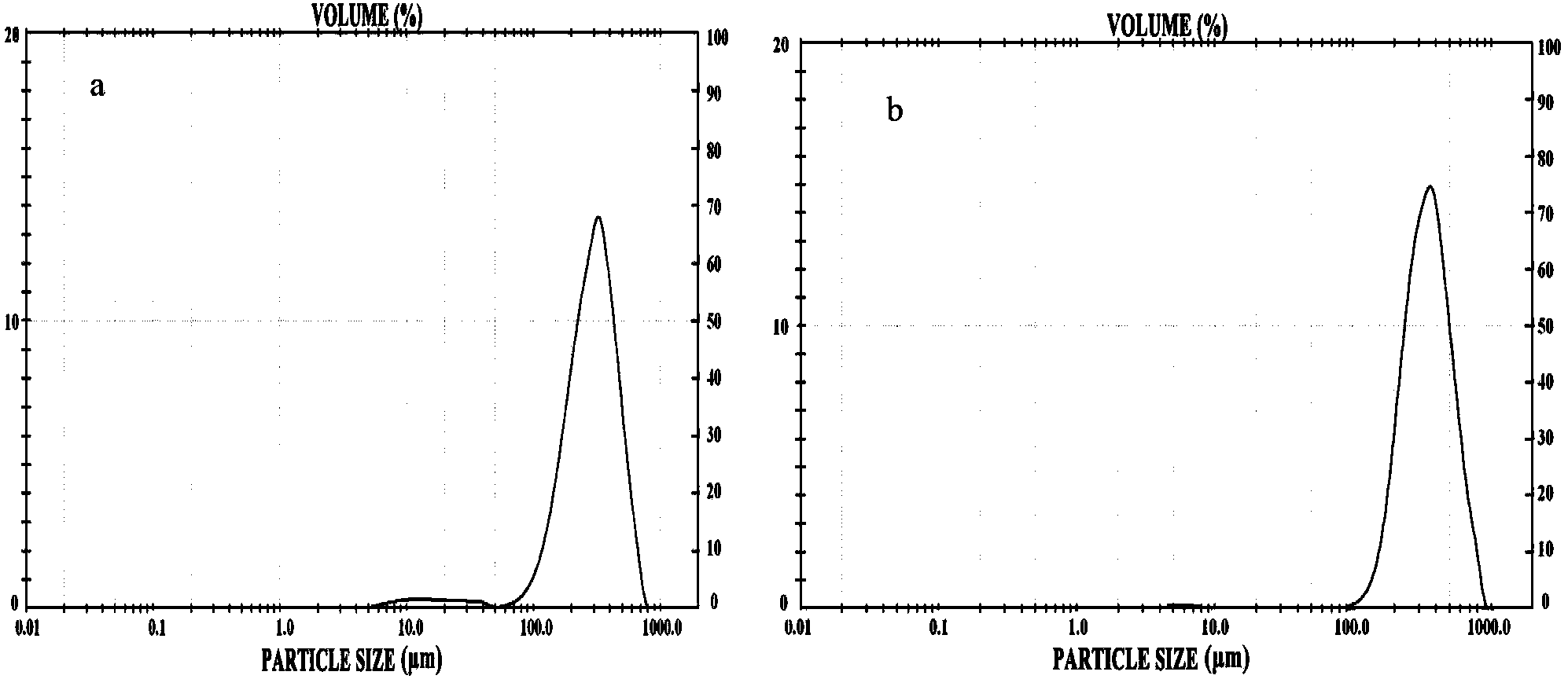
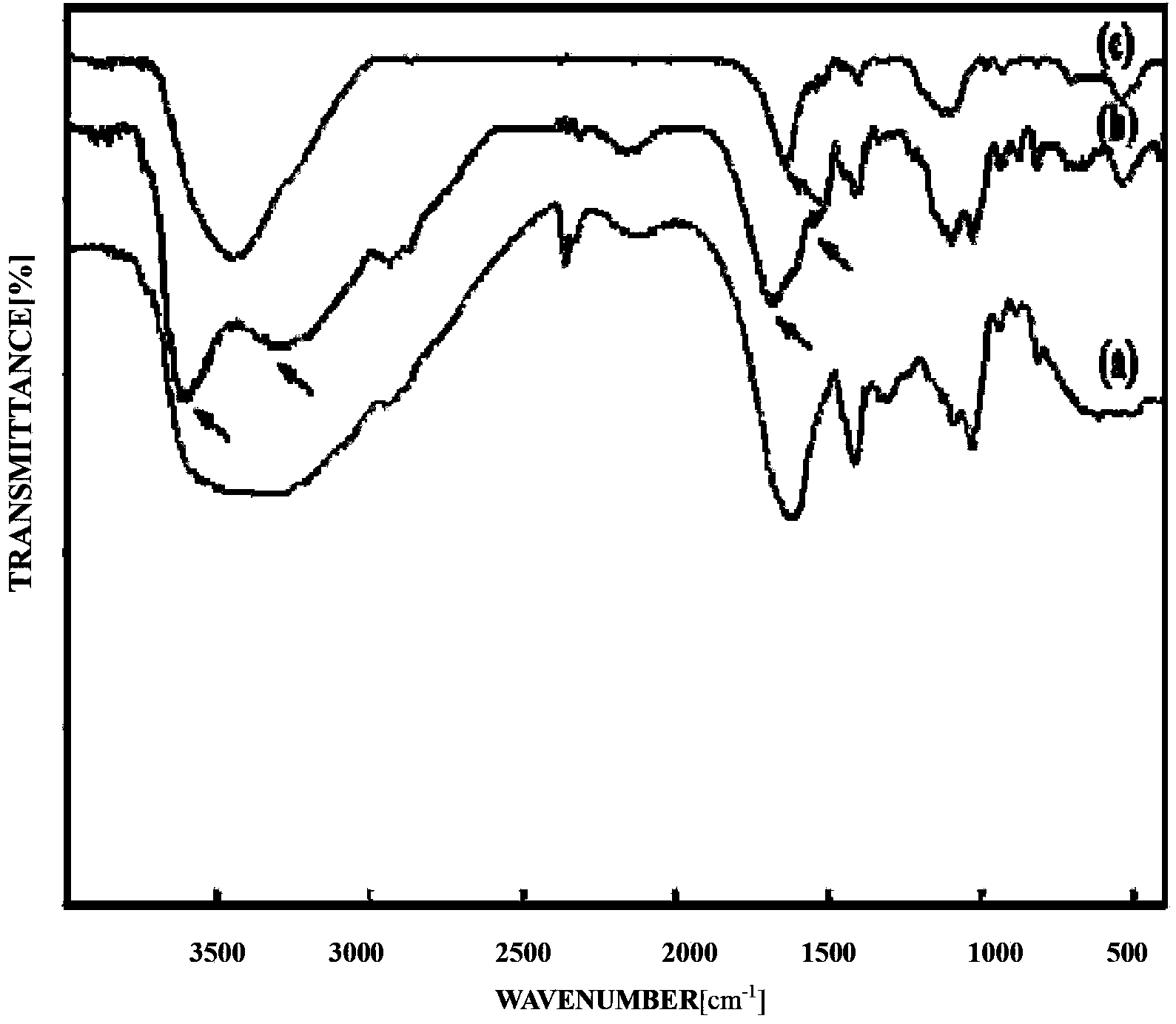
Biological adhesive microsphere with nuclear shell structure and preparation method of microsphere
A technology of bioadhesion and core-shell structure, applied in the field of medicine, can solve the problem of low eradication rate of Helicobacter pylori, achieve the effect of reducing drug loss and good mucosal adhesion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] 1. Preparation of alkaloid ethanol solution
[0080] 1) Crush the raw materials Coptis chinensis and Evodia rutaecarpa, add them to an ethanol solution with a concentration of 60-75% by mass, heat and reflux for extraction 3-5 times, each time for 1.5-2 hours, wherein the weight ratio of Coptis chinensis and Evodia rutaecarpa is 6:1 , the ratio of the total weight of Coptidis Rhizoma and Evodia Fructus to the total volume of ethanol solution in each extraction process is 1:8-12 (g / ml or kg / L), that is, adding ethanol solution 8 -12ml or add 8-12L of ethanol solution to every 1kg of Coptis rhizome and Evodia rutaecarpa mixed raw materials;
[0081] 2) Combine the reflux extracts, concentrate into an extract with a relative density of 1.10-1.30, then place the extract in a freeze dryer (Savant Modulyo D freeze dryer, Thermo company, U.S.) to freeze-dry to constant weight, The total alkaloid extract was obtained.
[0082] In the present invention, besides 6:1, the weight...
Embodiment 2
[0144] 1. In the process of preparing the microsphere core, except that it is different as described below, all the other are the same as in Example 1, specifically as follows:
[0145] 1) The volume ratio of emulsifier Span80 and Tween80 in the mixed emulsion preparation step is 3:1; the ratio of the total volume of emulsifier Span80 and Tween80 to the volume of liquid paraffin is 1:90; the stirring temperature is 50°C;
[0146] 2) The weight ratio of gelatin and sodium alginate in the first carrier solution preparation step is 6:4, and the ratio of the total weight of gelatin and sodium alginate to the volume of the aqueous solution is 10:100 (g / ml), that is, every 100ml The total weight of gelatin and sodium alginate added in the water is 10g;
[0147] 3) In the emulsification treatment step:
[0148] The temperature of the first emulsification treatment is 35°C, the stirring speed is 500rpm, the drop rate of the gelatin-sodium alginate aqueous solution into the mixed emul...
Embodiment 3
[0161] 1. In the process of preparing the microsphere core, except that it is different as described below, all the other are the same as in Example 1, specifically as follows:
[0162] 1) The volume ratio of emulsifier Span80 and Tween80 in the mixed emulsion preparation step is 5:1; the ratio of the total volume of emulsifier Span80 and Tween80 to the volume of liquid paraffin is 1:110; the stirring temperature is 40°C;
[0163] 2) The weight ratio of gelatin and sodium alginate in the first carrier solution preparation step is 8:2, and the ratio of the total weight of gelatin and sodium alginate to the volume of the aqueous solution is 5:100 (g / ml), that is, every 100ml The total weight of the gelatin and sodium alginate added in the water is 5g;
[0164] 3) In the emulsification treatment step
[0165] The temperature of the first emulsification treatment is 55°C, the stirring speed is 300rpm, and the gelatin-sodium alginate aqueous solution is dripped
[0166] The rate ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com