Preparation method of magnesium fluoride with high specific surface area
A magnesium fluoride and magnesium source technology, applied in the direction of magnesium fluoride and magnesium halide, can solve the problems of low specific surface area of magnesium fluoride, inconvenient operation and control, complicated preparation process, etc., achieve high specific surface area, easy control, The effect of simple equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
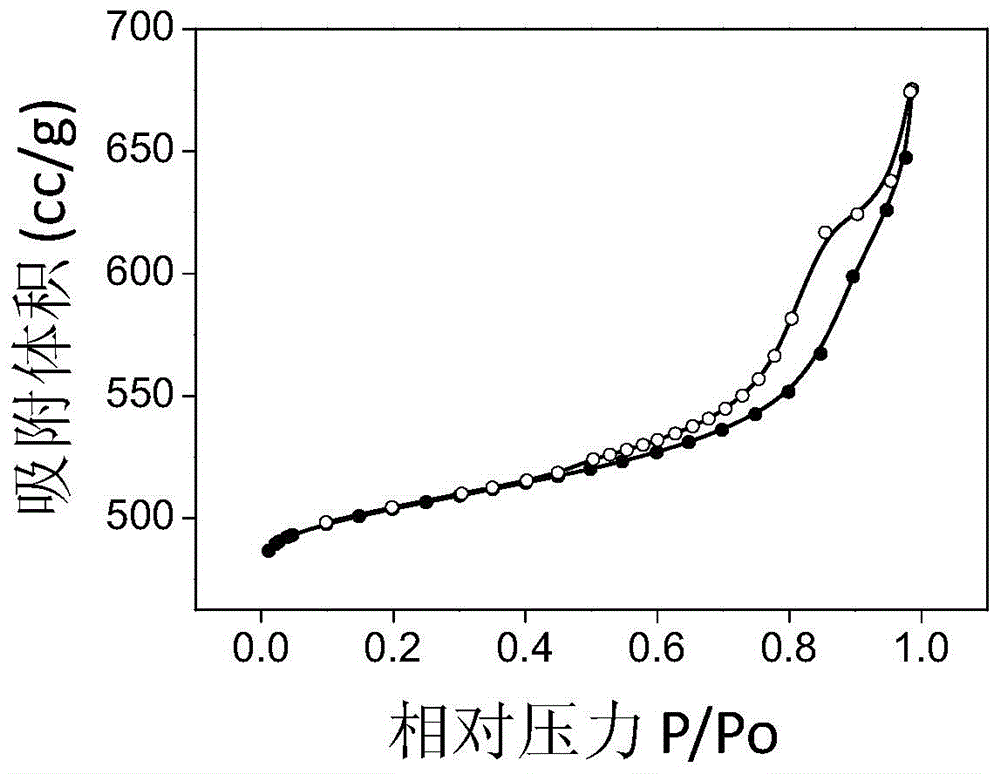
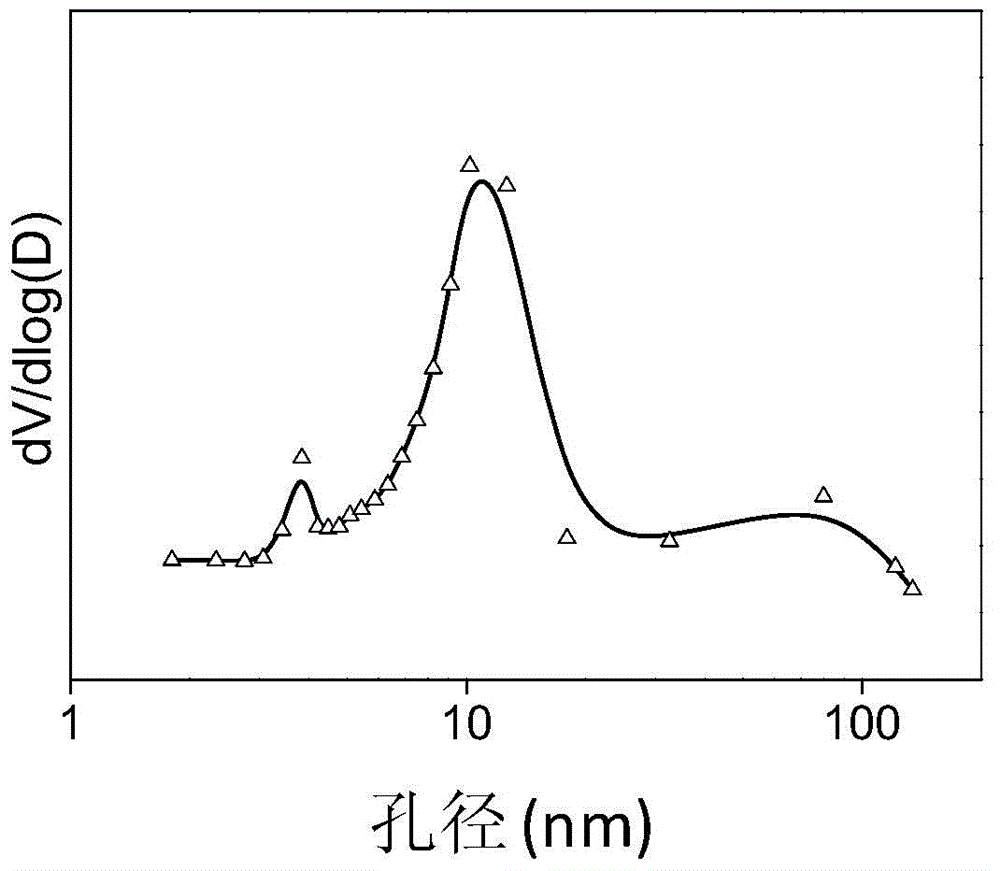
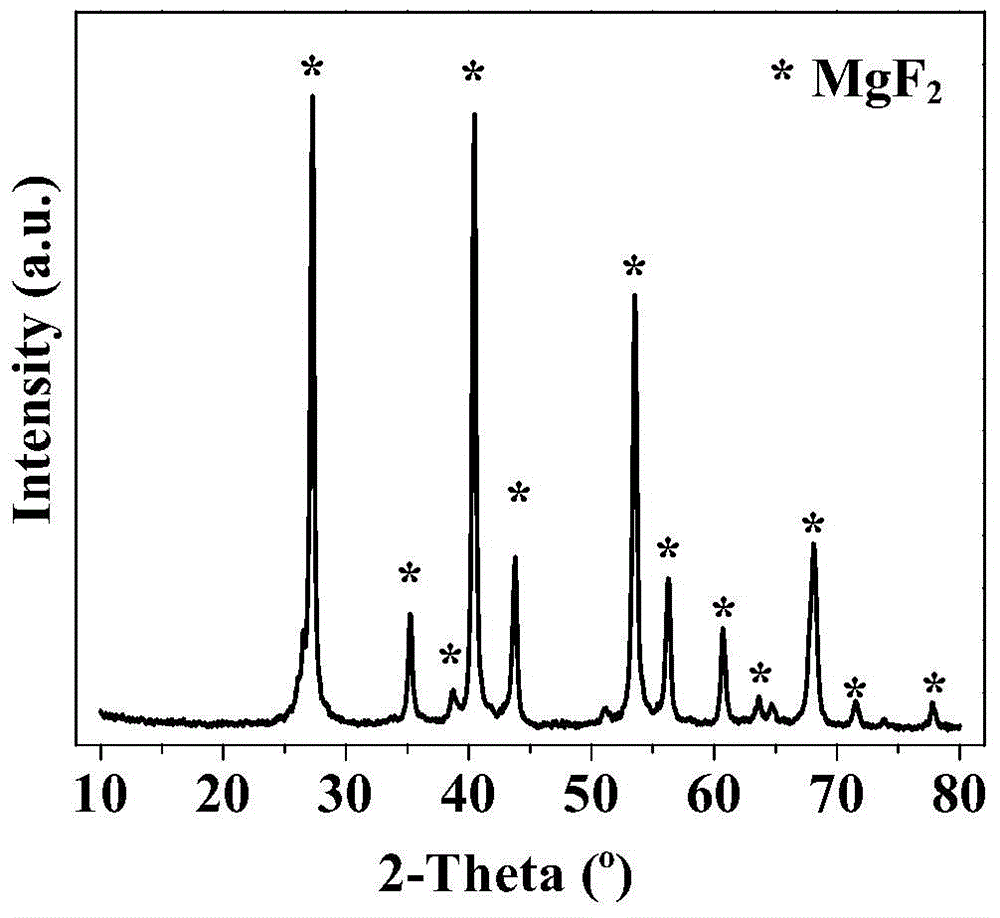
[0036] Prepare 100ml equal volume of 2mol / L NH 4 F solution and 1mol / L Mg(CH 3 COO) 2 4H 2 O solution, and sucrose (containing C 0.4mol) was added to the magnesium acetate solution, and the sucrose was completely dissolved. Mix by positive addition, and turn on magnetic stirring at the same time, react at a temperature of 30 °C for 7 hours, then leave it for aging at a constant temperature for 2 hours, evaporate the resulting solution to dryness, and put it in an oven at 100 °C for pre-carbonization for 6 hours, and then Pre-carbonize in an oven at 160°C for 6 hours, then bake in a muffle furnace under a nitrogen atmosphere at 400°C for 4 hours to obtain charcoal-containing magnesium fluoride, and finally bake in a muffle furnace under an air atmosphere at 400°C for 4 hours to remove carbon Template, you can get magnesium fluoride with high specific surface area, its specific surface area is 184m 2 / g, the pore volume is 0.35cc / g, and the most probable pore size distribution...
Embodiment 2
[0039] Prepare each 100ml equal volume of 2mol / L NaF solution and 1mol / L Mg(CH 3 COO) 2 4H 2 O solution, and sucrose (containing C 0.1mol) was added to the magnesium acetate solution, and the sucrose was completely dissolved. Mix by positive addition, and turn on magnetic stirring at the same time, react at a temperature of 30 °C for 7 hours, then leave it for aging at a constant temperature for 2 hours, evaporate the resulting solution to dryness, and put it in an oven at 100 °C for pre-carbonization for 6 hours, and then Pre-carbonize in an oven at 160°C for 6 hours, then bake in a muffle furnace under a nitrogen atmosphere at 400°C for 4 hours to obtain carbon-containing magnesium fluoride, and finally bake in a muffle furnace under an air atmosphere at 400°C for 3 hours to remove carbon Template, you can get magnesium fluoride with high specific surface area, its specific surface area is 73m 2 / g.
Embodiment 3
[0041] Prepare 100ml equal volume of 2mol / L NH 4 F solution and 1 mol / L MgC 2 o 4 .2H 2 O solution, and sucrose (containing C 1mol) was added to the ammonium fluoride solution, and the sucrose was completely dissolved. Mix by reverse addition, and turn on magnetic stirring at the same time, react at a temperature of 30°C for 7 hours, then leave it for aging at a constant temperature for 2 hours, evaporate the resulting solution to dryness, and put it in an oven at 100°C for pre-carbonization for 6 hours, and then Pre-carbonize in an oven at 160°C for 6 hours, then bake in a muffle furnace under a nitrogen atmosphere at 400°C for 4 hours to obtain charcoal-containing magnesium fluoride, and finally bake in a muffle furnace under an air atmosphere at 400°C for 12 hours to remove carbon Template, you can get magnesium fluoride with high specific surface area, its specific surface area is 153m 2 / g.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com