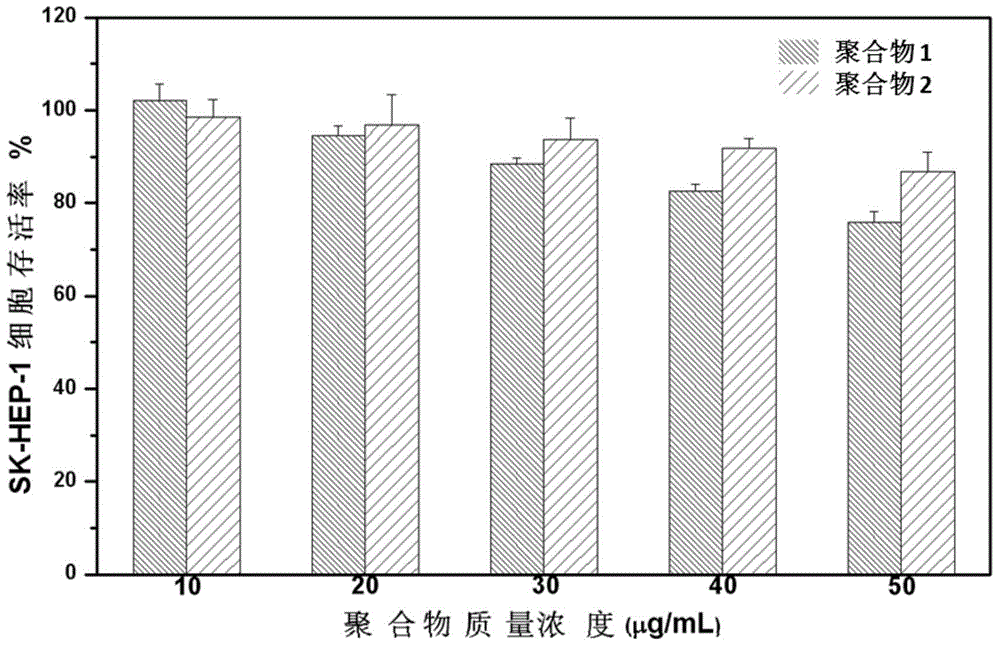
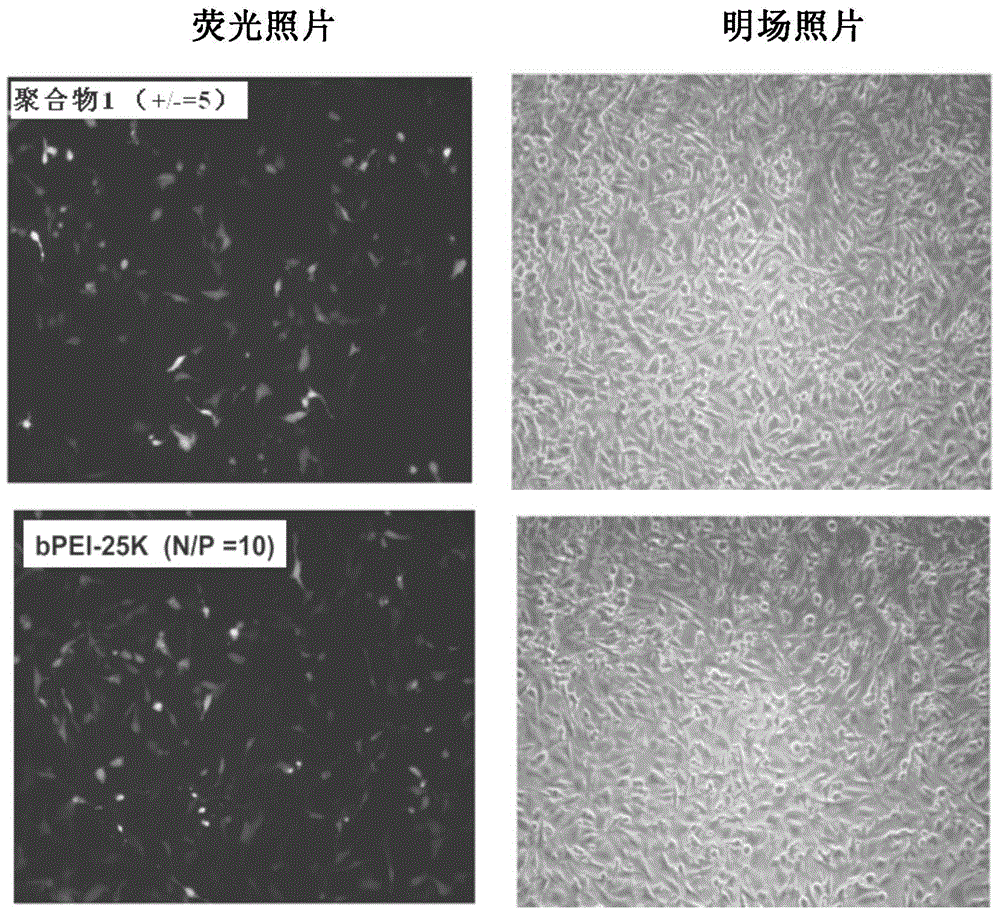
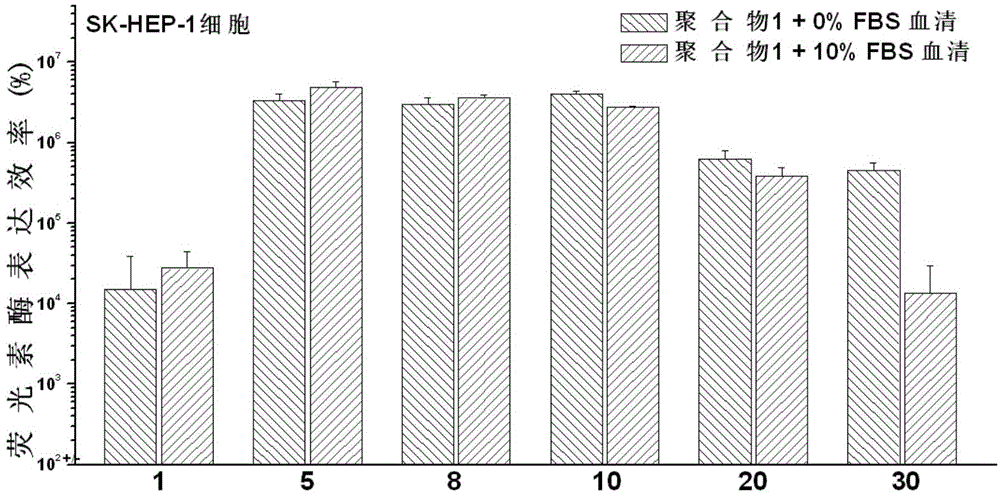
Polymethacrylamide cationic polymer cooperatively modified by side groups natural arginine and lactobionic acid, preparation method and application
A technology of polymethacrylamide cation and polymethacrylamide, which is applied in the field of biological functional materials, can solve the problems of reducing cell membrane damage ability and cationic charge density, and achieves reduced interaction and surface adsorption, low cytotoxicity, Effect of increasing surface osmotic pressure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The first step: the RAFT reagent 4-cyano-4-(dodecyltrithiocarbonic acid) valeric acid (35 mg), the hexamethylenediaminomethacrylamide functional monomer protected by the terminal amino Boc Azobisisobutyronitrile AIBN (2.8 mg) free radical initiator and 6 mL of dry tetrahydrofuran solvent were added to the Schlenk reaction tube. After 3 cycles of freezing-vacuumizing-thawing and nitrogen circulation to remove residual oxygen in the reaction solution, keep the reaction tube under a nitrogen atmosphere, move it into an oil bath preheated to 70°C and stir for 6 hours. Then the reaction was rapidly quenched with liquid nitrogen, and the reaction solution was concentrated by rotary evaporation under reduced pressure. The concentrated solution was precipitated in glacial ether to obtain a light yellow solid, which was filtered and dried in a vacuum oven for 24 hours to prepare hexamethylenediamine-terminated amino-protected Boc The methacrylamide polymer, conversion rate is 6...
Embodiment 2
[0033] The first step: the RAFT reagent 4-cyano-4-(dodecyltrithiocarbonic acid) valeric acid (70mg), the hexamethylenediaminomethacrylamide functional monomer protected by the terminal amino Boc Azobisisobutyronitrile AIBN (5.6 mg) free radical initiator and 15 mL of dry toluene solvent were added to the Schlenk reaction tube. After 3 cycles of freezing-vacuumizing-thawing and nitrogen circulation to remove residual oxygen in the reaction solution, keep the reaction tube under a nitrogen atmosphere, move it into an oil bath preheated to 70°C and stir for 6 hours. Then the reaction was rapidly quenched with liquid nitrogen, and the reaction solution was concentrated by rotary evaporation under reduced pressure. The concentrated solution was precipitated in glacial ether to obtain a light yellow solid, which was filtered and dried in a vacuum oven for 24 hours to prepare hexamethylenediamine-terminated amino-protected Boc The methacrylamide polymer, the conversion rate is 68%. ...
Embodiment 3
[0040] The first step: the RAFT reagent 4-cyano-4-(dodecyltrithiocarbonic acid) valeric acid (35 mg), the hexamethylenediaminomethacrylamide functional monomer protected by the terminal amino Boc Azobisisobutyronitrile AIBN (2.8 mg) free radical initiator and 6 mL of dry tetrahydrofuran solvent were added to the Schlenk reaction tube. After 3 cycles of freezing-vacuumizing-thawing and nitrogen circulation to remove residual oxygen in the reaction solution, keep the reaction tube under a nitrogen atmosphere, move it into an oil bath preheated to 70°C and stir for 6 hours. Then the reaction was rapidly quenched with liquid nitrogen, and the reaction solution was concentrated by rotary evaporation under reduced pressure. The concentrated solution was precipitated in glacial ether to obtain a light yellow solid, which was filtered and dried in a vacuum oven for 24 hours to prepare hexamethylenediamine-terminated amino-protected Boc The methacrylamide polymer, conversion rate is 6...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com