Degradable endocranium repair stent compounded by human amniotic membrane and bull dorsal aponeurosis and preparation method of repair stent
A technology of human amniotic membrane and dura mater, applied in medical science, prosthesis, etc., can solve the problems of lax application, cerebrospinal fluid leakage, production process, production cost and commodity circulation cost, etc., and achieve biological safety improvement, Increased biological security, the effect of retaining biological properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] A preparation method for a dura mater repair bracket compounded with degradable human amniotic membrane and bovine dorsal tendon, comprising the following steps:
[0036] (1) Preparation of amnion: According to the national standard (YZB / National 0593-2005), the amnion is obtained and prepared; in the production of human amnion, the amnion epithelial cells can be retained or the epithelial cells can be removed by enzymatic hydrolysis;
[0037] (2) Preparation of beef back tendon slices: use repeated freezing and thawing to make the cells in the material form ice crystals, increase the salt concentration of the remaining cytosol and cause the cells to swell and break, and change the material and cell surface antigens to significantly reduce the antigen of the biological material sex. Studies have shown that repeated freezing and thawing combined with enzyme treatment of 0.3mm tendon thin slices has a good decellularization effect, collagen will not disintegrate, and the ...
Embodiment 1
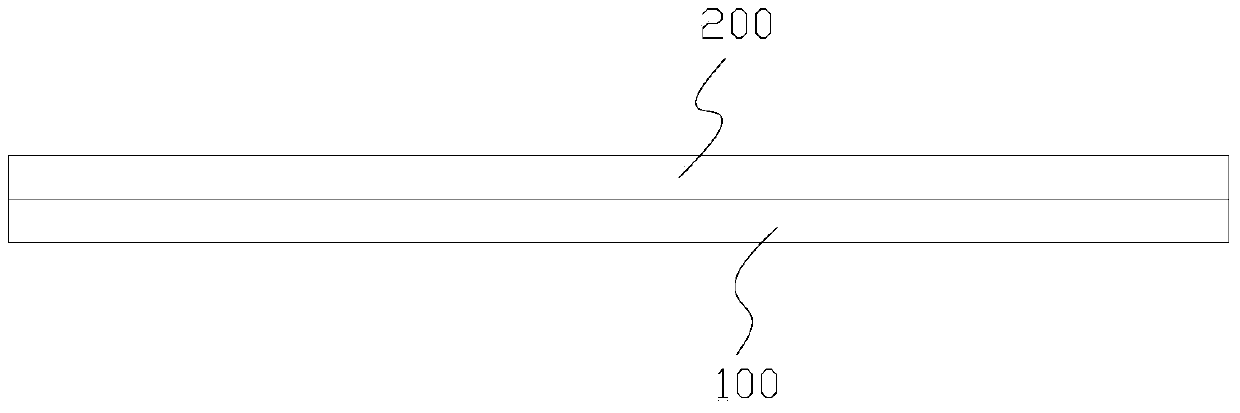
[0047] see figure 1 , a degradable dural repair scaffold composed of human amniotic membrane and bovine dorsal tendon, comprising the amnion 100 prepared above and bovine dorsal tendon sheet 200, the epithelial layer of the amniotic membrane 100 is laid down, and then the treated bovine The dorsal tendon sheet 200 is laid on the amniotic membrane 100, and the dura mater repair bracket is obtained by compounding the human amniotic membrane and the bovine dorsal tendon sheet through non-toxic cross-linking.
Embodiment 2
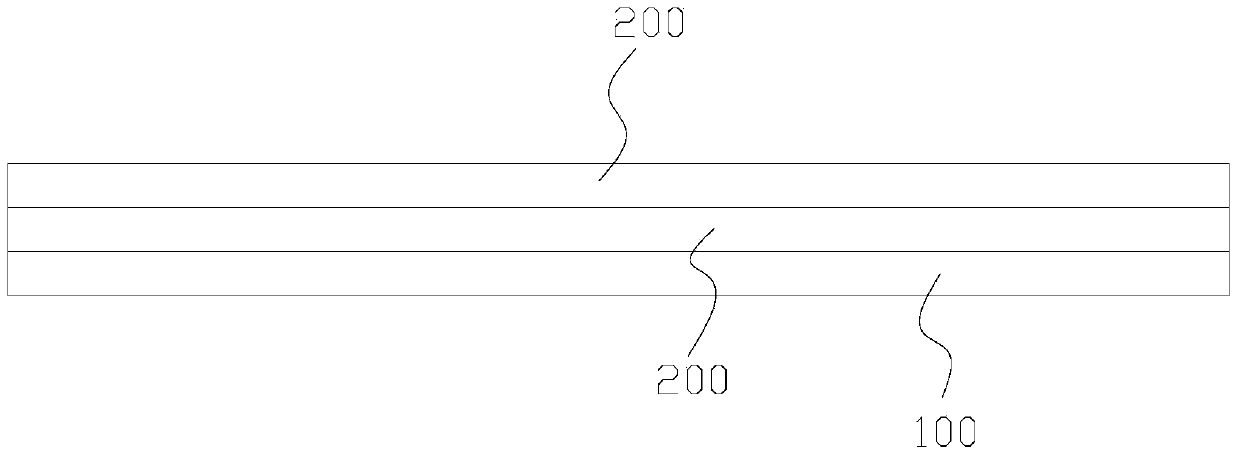
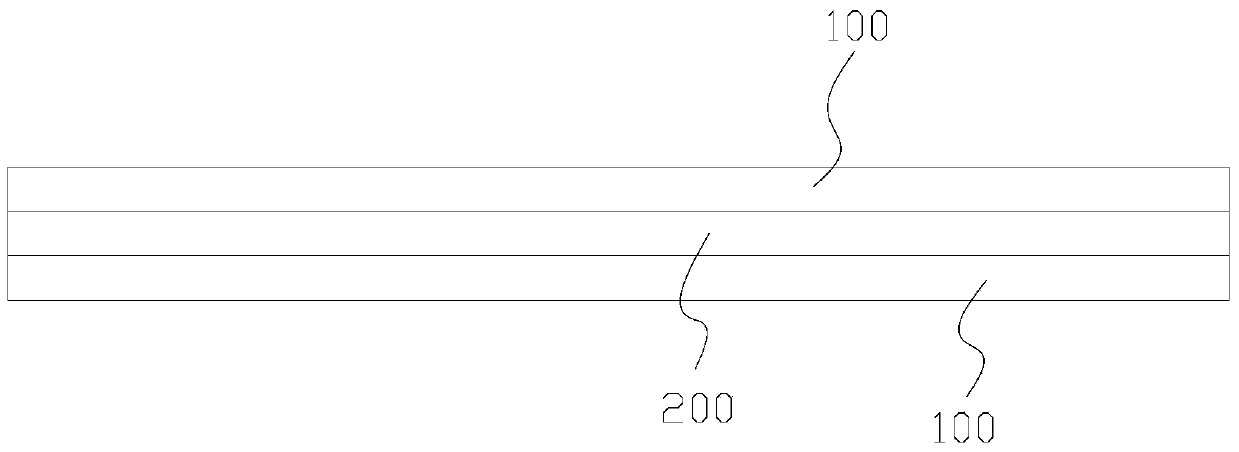
[0049] see figure 2 , a dural repair scaffold composed of degradable human amniotic membrane and bovine dorsal tendon, comprising the amnion 100 prepared above and bovine dorsal tendon sheet 200, the bovine dorsal tendon sheet 200 includes two layers, and the two-layer bovine dorsal tendon sheet 200 according to The tendon collagen fibers are stacked horizontally and vertically, and then the stacked two-layer bovine dorsal tendon sheet 200 is laid on the amniotic membrane 100, and the human amniotic membrane obtained through nontoxic cross-linking and the bovine dorsal tendon sheet are composited Dural repair stents. ( figure 2 There is also a case of adding a layer of amniotic membrane on top,)
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Length | aaaaa | aaaaa |
| Width | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com