Polypeptide targeting human breast cancer cells and application of polypeptide
A human breast cancer cell and targeting technology, applied in the direction of application, medical preparations of non-active ingredients, peptides, etc., can solve the problems of cumbersome preparation of antibody drugs, weak penetration, poor stability, etc., and achieve non-immunogenicity , strong selectivity and small molecular weight
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
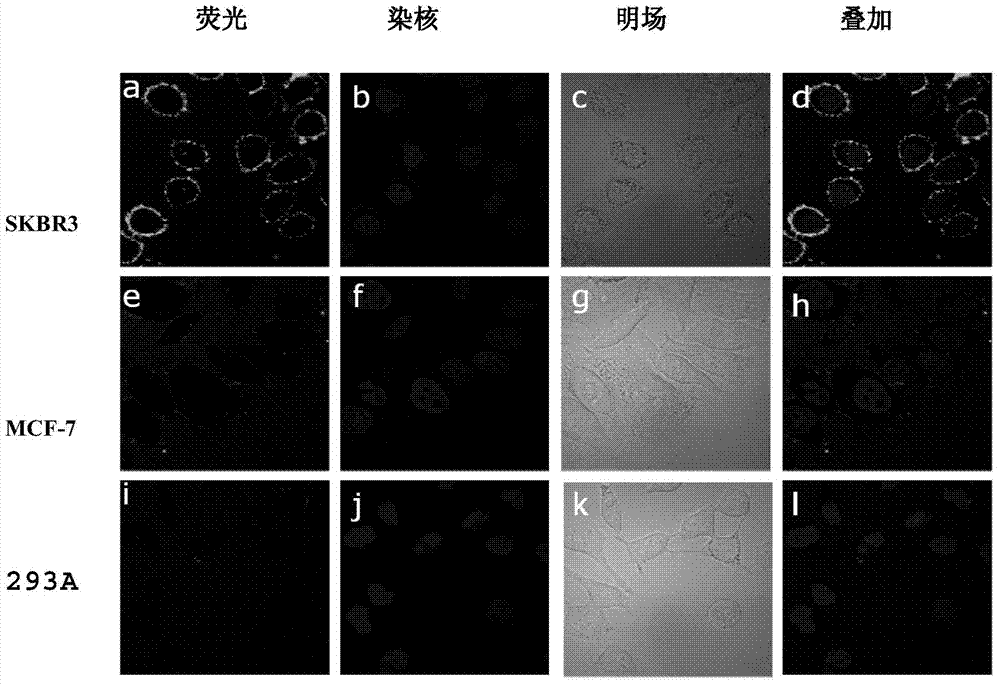
Examples
Embodiment 1
[0054] Embodiment 1 Construction of the polypeptide screening system of the present invention
[0055] 1. Experimental instruments and materials
[0056] N-methylmorpholine (NMM), piperidine, trifluoroacetic acid (TFA), dichloromethane (DCM), ninhydrin, vitamin C, phenol, tetramethyluronium hexafluorophosphate (HBTU), hexahydro Pyridine, triisopropylsilane (TIS), ethanedithiol (EDT), N,N dimethylformamide (DMF), anhydrous ether, resin, methanol, various Fmoc protected amino acids, peptide synthesis tubes, shaking Bed, vacuum water pump, rotary evaporator, above reagents and materials were obtained from commercial sources.
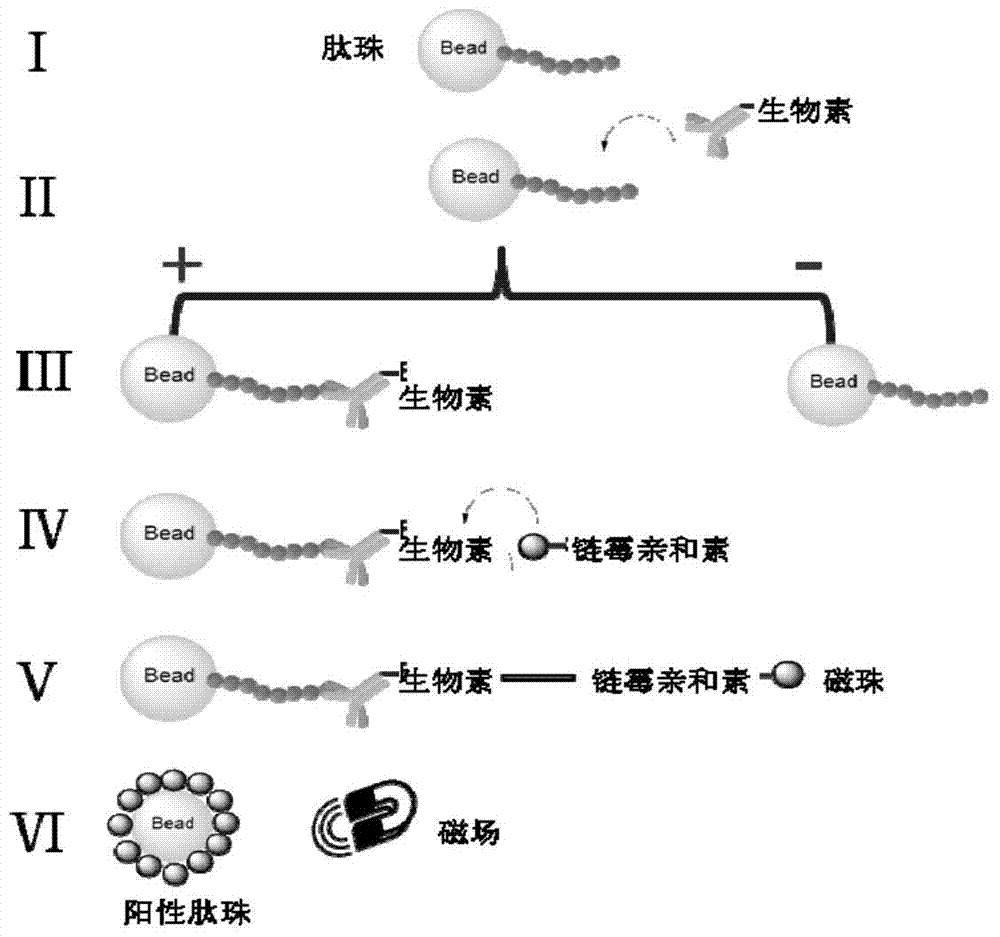
[0057] 2. Synthesis of "one bead, one object" polypeptide library
[0058] Using the Fmoc solid-phase peptide synthesis method to synthesize a peptide library, the process of screening HER2-targeted peptides is as follows: figure 1 shown. The specific method is to couple the protected amino acids to the solid phase resin one by one, and then cleave the ...
experiment example 1
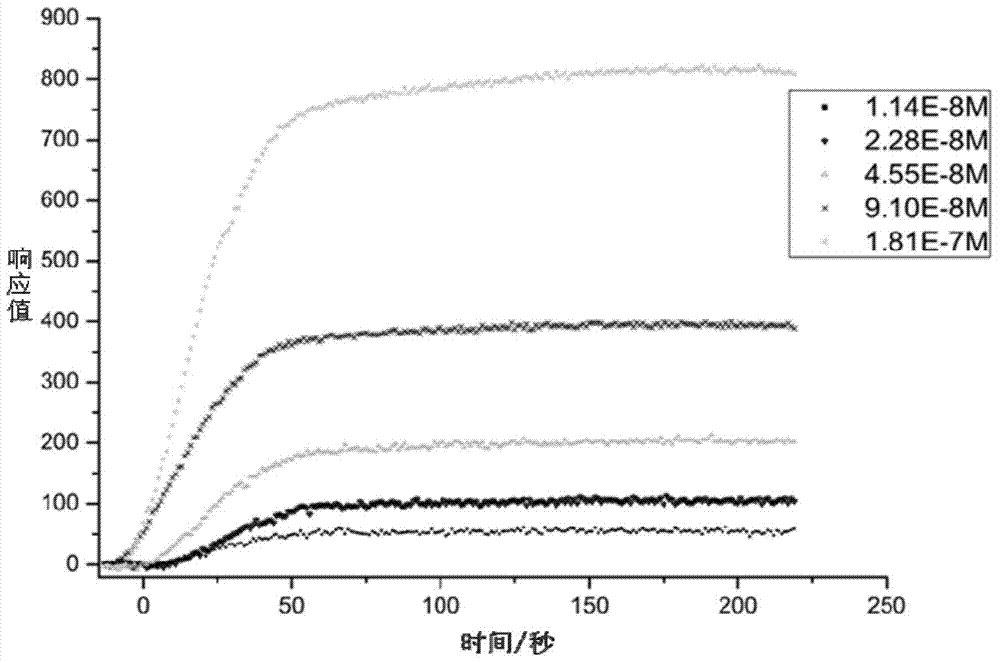
[0065] Experimental Example 1 Detection of affinity between H10F and H6F polypeptides and HER2 protein by surface plasmon resonance (SPRi) method
[0066] Spot 1 mg / mL H10F and H6F peptides and 1×PBS on the chip, incubate overnight at 4°C under humid conditions, then wash with 10×PBS for 10 minutes, then with 1×PBS for 10 minutes, and finally wash with deionized water for 2 minutes. Once, 10min each time, immersed in 1×PBS containing 5% milk, incubated overnight at 4°C, then washed with 10×PBS for 10min, 1×PBS for 10min, and finally washed twice with deionized water, 10min each time , blown dry with nitrogen, and put the chip on the machine (Plexera HT surface plasmon resonance imaging system).
[0067] The mobile phase was sequentially passed through 1×PBS, 2×PBS, 1.25 μg / mL, 2.5 μg / mL, 5 μg / mL, 10 μg / mL and 20 μg / mL human HER2 purified protein, and the SPRi signal was recorded and analyzed.
[0068] Depend on figure 2 It can be seen that the SPRi signals of H10F and H6F...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com