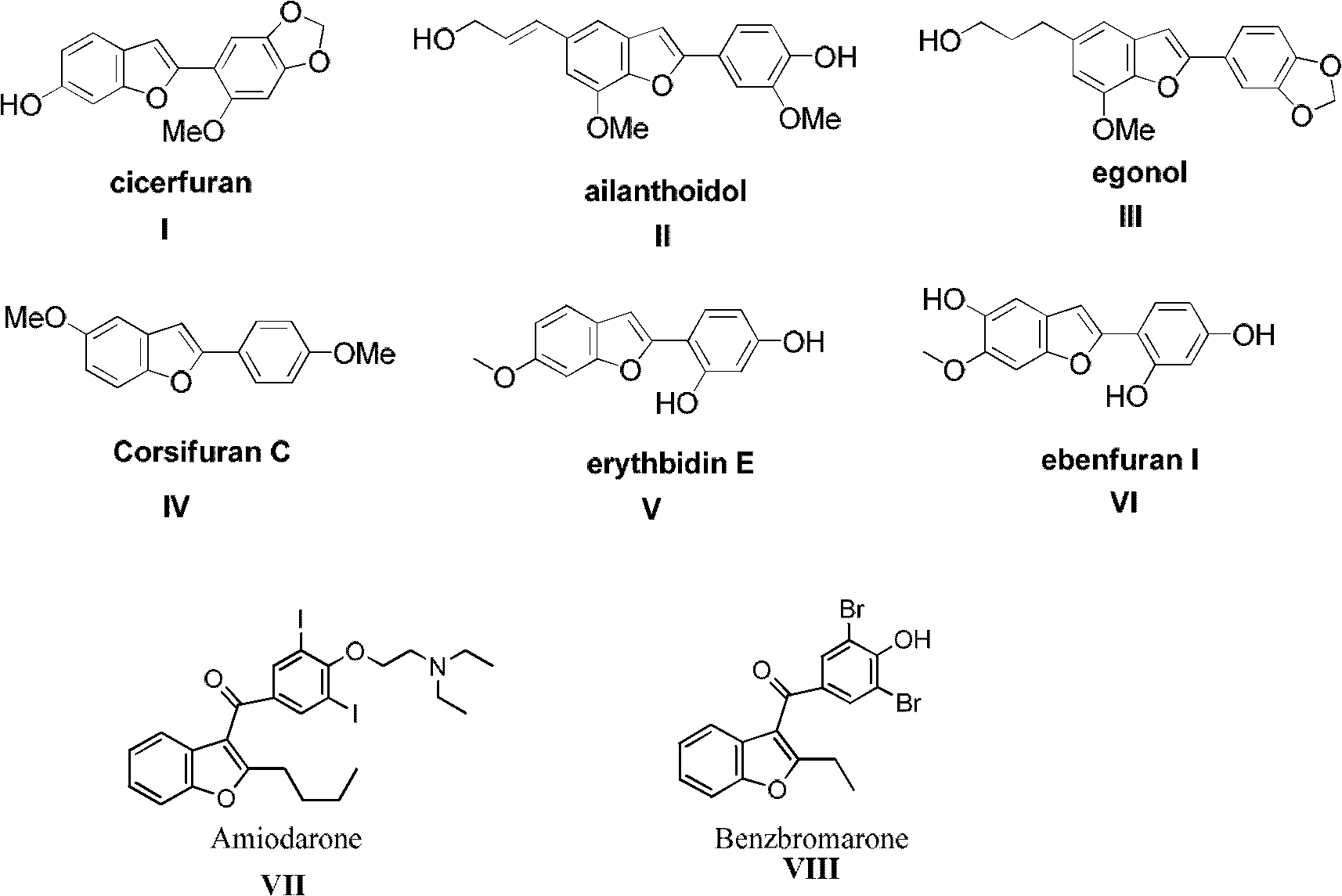
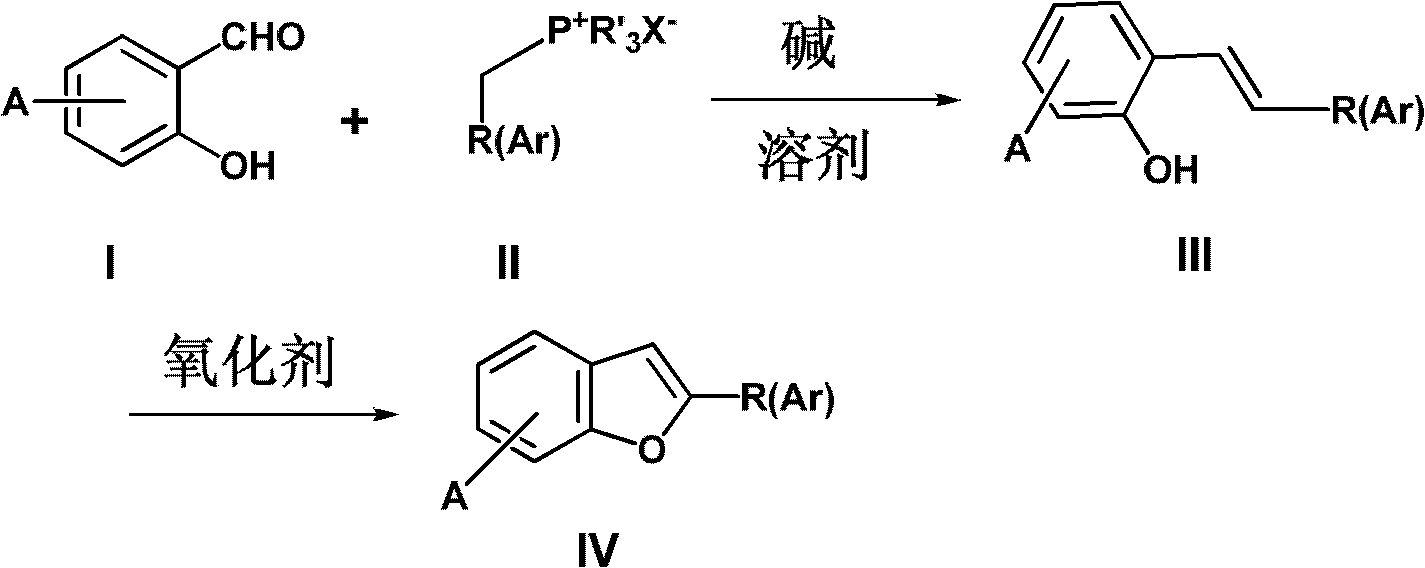
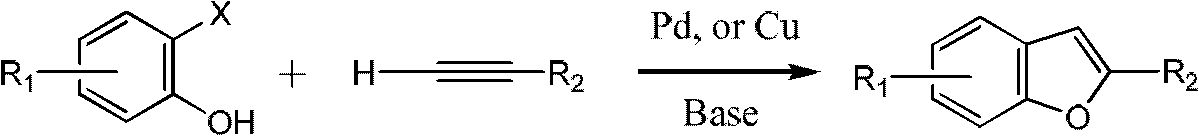Method for synthesizing benzofuran derivatives in one pot process
A technology for benzofuran and derivatives, which is applied in the field of one-pot synthesis of benzofuran derivatives, can solve the problems of high price, inapplicability of halogen-containing benzofuran, and limited sources of raw materials, and achieve low cost and applicable Wide Range of Selective Effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1, Preparation of 2-(3,4-dimethoxyphenyl)benzofuran
[0030]
[0031] Salicylaldehyde (0.61g, 5mmol), 3,4-dimethoxybenzyltriphenylphosphonium chloride (2.5g, 5.5mmol), potassium hydroxide (0.34g, 6.0mmol) were added to 50mL of toluene in sequence , Stir the reaction at room temperature (25°C) until the salicylaldehyde disappears substantially or completely. Add powdered anhydrous potassium carbonate (3.5g, 25.0mmol) to the system, stir for half an hour, if stirring is difficult, add toluene or tetrahydrofuran, add iodine (6.4g, 25.0mmol), stir at room temperature (25℃) until the reaction complete. 80mL saturated sodium bicarbonate solution was added to the reaction mixture, and protective sodium bisulfite solution was added dropwise until the color of the reaction solution faded. The resulting mixture was extracted with ethyl acetate (3×80mL), the organic layer was dried over anhydrous sodium sulfate, and the solvent was recovered The obtained crude product was p...
Embodiment 2
[0033] Example 2, 2-(3-Methoxy-4-benzyloxyphenyl)-6-methoxybenzofuran
[0034]
[0035] 4-methoxysalicylic aldehyde (0.76g, 5mmol), 3-methoxy-4-benzyloxybenzyltriphenylphosphonium chloride (2.9g, 5.5mmol), potassium hydroxide (0.70g, 12.5 mmol) was sequentially added to a thoroughly dried mortar, ground vigorously at room temperature (25°C) for 30 minutes, and then intermittently ground for 2 to 3 hours. The resulting paste was transferred to a 250 mL three-necked reaction flask, and 20 mL of tetrahydrofuran (or After washing the residue with toluene, transfer it to a reaction flask, add tetrahydrofuran (or toluene) to 50 mL, and stir and react at room temperature (25° C.) for 2 to 3 hours. Add powdered anhydrous potassium carbonate (3.5g, 25.0mmol) to the system, stir for half an hour, if stirring is difficult, add toluene or tetrahydrofuran, add iodine (6.4g, 25.0mmol), stir at room temperature (25℃) until the reaction complete. 80mL saturated sodium bicarbonate solution was ...
Embodiment 3
[0041] Example 3. Methyl 4-(7-methoxy-2-benzofuranyl)benzoate
[0042]
[0043] 3-Methoxysalicylic aldehyde (0.76g, 5mmol), 4-formate methylbenzyltriphenylphosphonium chloride (2.5g, 5.5mmol), potassium hydroxide (0.6g, 6.0mmol) were added to In 50 mL of toluene, the reaction was stirred at room temperature (25° C.) until the 3-methoxy salicylaldehyde disappeared substantially or completely. Add glacial acetic acid (0.40g, 6mmol) dropwise to the system, add dichlorodicyanobenzoquinone (DDQ) (1.7g, 7.5mmol), and heat to reflux until the reaction is complete. 80 mL of saturated sodium bicarbonate solution was added to the reaction mixture, the resulting mixture was extracted with ethyl acetate (3×80 mL), the organic layer was dried over anhydrous sodium sulfate, the solvent was recovered, and the crude product obtained was purified by column chromatography to obtain the target product 4-(7 -Methoxy-2-benzofuryl) benzoic acid methyl ester, yield: 69%.
[0044] The prepared product w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com