Acridine-1, 3, 4-thiadiazole type compound and preparation method and application thereof
A technology of thiadiazoles and compounds is applied in the field of medicine to achieve the effects of improving anti-tumor activity, increasing the conjugated area and significantly in vitro anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
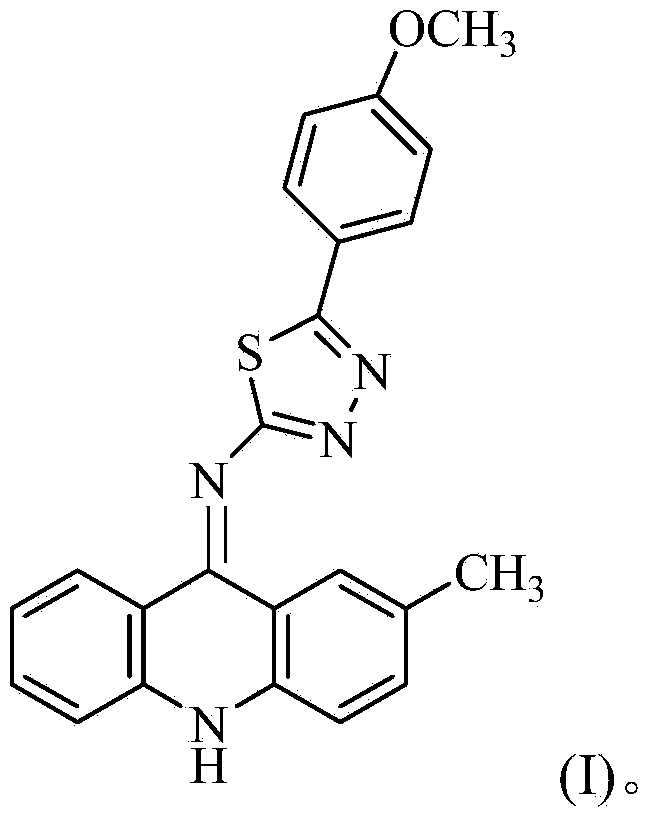
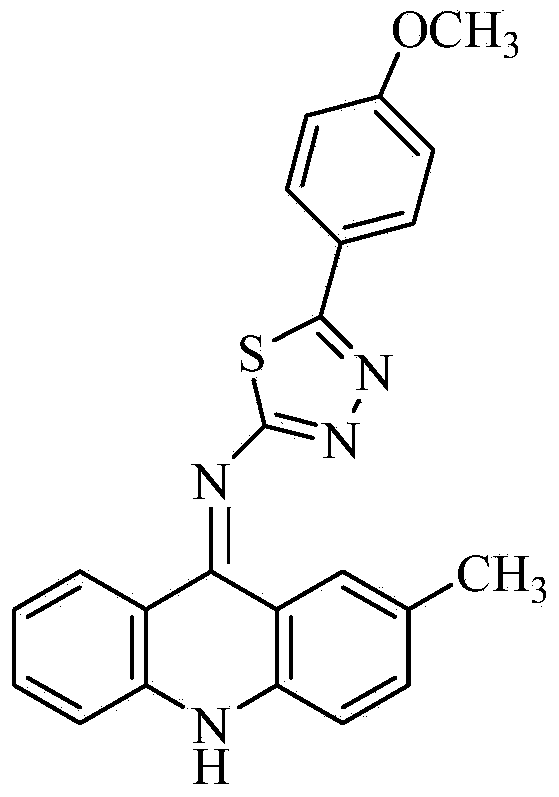
[0027] The preparation of 2-(2-methyl-9-acridinimino)-3-p-methoxyphenyl-1,3,4-thiadiazole, the specific steps are as follows:
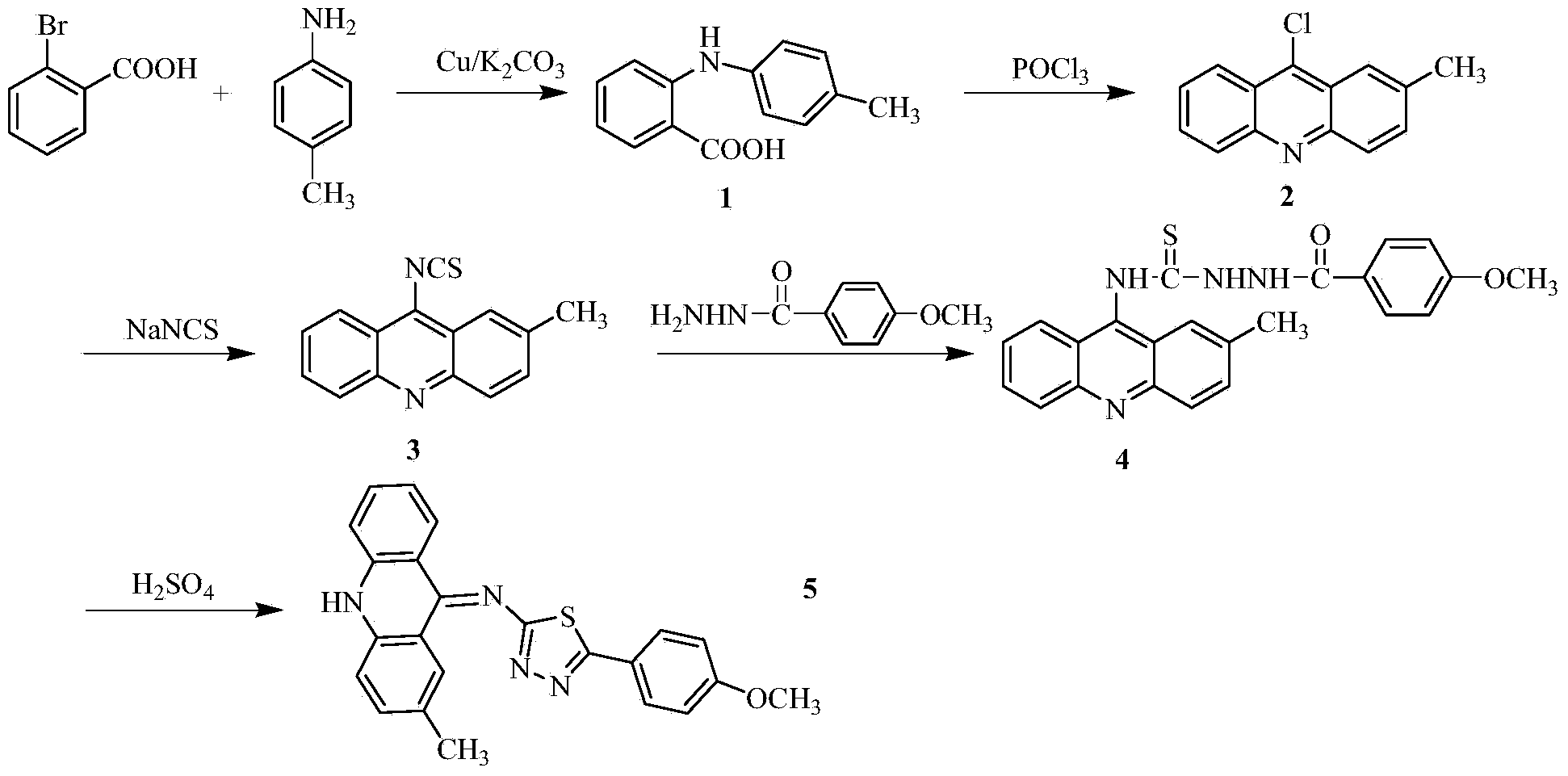
[0028] 1) In a 250ml three-necked flask, add 5.20g (26mmol) of o-bromobenzoic acid, 3.64g (34mmol) of p-methylaniline, 7.5g (36.2mmol) of potassium carbonate and 0.3g (4.7mmol) of copper powder, and then add 30ml of isoamyl alcohol was used as a solvent, and stirred under reflux at 140°C for 2h. After the reaction, evaporate the solvent under reduced pressure, add 600ml of water to the obtained residue, react at 80°C for 20 minutes, filter while hot, wash the filter cake, combine the water layer, acidify the water layer with concentrated hydrochloric acid to pH=2, and precipitate a large amount of light green Precipitation, suction filtration, and the obtained solid was recrystallized with chloroform to obtain compound 1 with a yield of 79%;
[0029] 2) In a 100ml round bottom flask, add 14.38g (18mmol) of the compound and 14.37ml of phosphorus oxych...
Embodiment 2
[0039] Repeat Example 1, the difference is:
[0040] In step 1), change the consumption of potassium carbonate to 5.39g (26mmol), copper powder 0.17g (2.6mmol), replace isoamyl alcohol with n-amyl alcohol, change the temperature during reflux to 150°C, and combine the water The layer was acidified to pH=1 with concentrated hydrochloric acid;
[0041] In step 3), the consumption of sodium thiocyanate is changed to 1.62g (20mmoL), the temperature during the reaction is changed to 80°C, and the organic solvent acetone is replaced with methanol;
[0042] In step 4), acetonitrile is used to replace the organic solvent absolute ethanol, and the temperature at reflux is changed to 60°C;
[0043] In step 5), 98% concentrated sulfuric acid was replaced with acetic acid.
[0044] The isolated orange-yellow solid was analyzed by mass spectrometry and carbon spectrometry, and it was determined to be 2-(2-methyl-9-acridinimino)-3-p-methoxyphenyl-1,3,4-thiadiene azole.
Embodiment 3
[0046] Repeat Example 1, the difference is:
[0047] In step 1), the temperature at reflux was changed to 160° C., and the combined aqueous layer was acidified to pH=4 with concentrated hydrochloric acid;
[0048] In step 3), the organic solvent acetone is replaced with a composition of methanol and acetonitrile (the volume ratio of methanol and acetonitrile is 1:1);
[0049] In step 4), the amount of p-methoxybenzohydrazide was changed to 0.99 g (6 mmol), and the organic solvent absolute ethanol was replaced with acetone.
[0050] The isolated orange-yellow solid was analyzed by mass spectrometry and carbon spectrometry, and it was determined to be 2-(2-methyl-9-acridinimino)-3-p-methoxyphenyl-1,3,4-thiadiene azole.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com