Boric acid fluorescence molecular probe as well as preparation method and application thereof
A fluorescent molecular probe, boric acid technology, used in chemical instruments and methods, material analysis by optical means, analysis of materials, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
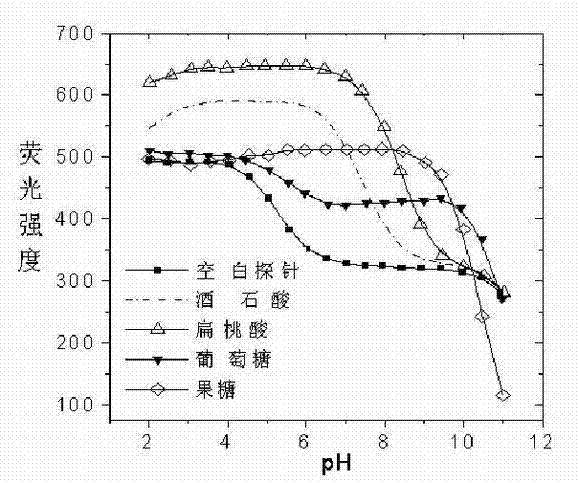
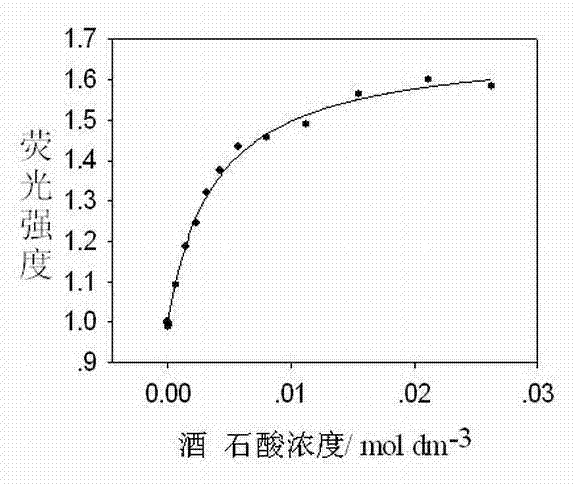
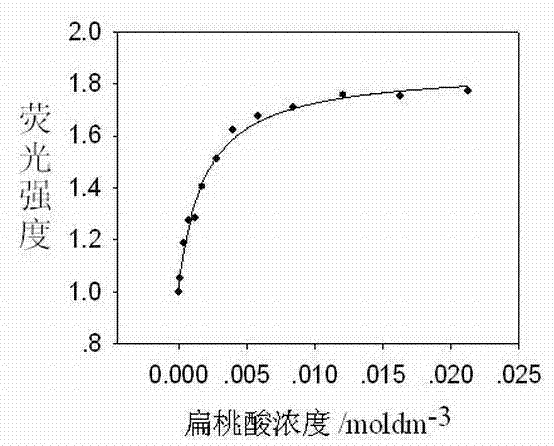
Image
Examples
Embodiment 1
[0047] Example 1 Fluorescent Probe Compounds 4 Synthesis:
[0048]
[0049] (1) compound 1 Synthesis
[0050] Add 2,4-dimethylpyrrole (3 ml, 30 mmol) and benzoyl chloride (2 mL, 18 mmol) into a 150 mL two-necked flask, and stir at room temperature for three days. Add triethylamine (13 mL, 90 mmol), BF 3 ×Et 2 O (15 mL, 120 mmol), stirred at room temperature for one day. The solvent was removed under reduced pressure. Separation by column chromatography (silica gel column, developing solvent is dichloromethane). A dark red sticky substance was obtained, which was recrystallized in methanol to obtain 800 mg of a red solid. Yield 13%.
[0051] (2) compound 2 Synthesis
[0052] In a 250 mL two-necked flask, ice-bathed and protected by argon, add 10 mL DMF and 10 mL phosphorus oxychloride. Stir at room temperature for 30 min. Add 100 mL dichloroethane, 1 (474 mg, 1.5 mmol), stirred at 50 °C for 3 h. The solvent was removed under reduced pressure, and the res...
Embodiment 2
[0057] Example 2 Fluorescent Probe Compounds 8 Synthesis:
[0058]
[0059] (2) compound 5 Synthesis
[0060] Add 2,4-dimethylpyrrole (3 ml, 30 mmol) and 4-diethylaminobenzoyl chloride (3.8 g, 18 mmol) into a 100 mL two-necked flask, and stir at room temperature for three days. Add triethylamine (13 mL, 90 mmol), BF 3 ×Et 2 O (15 mL, 120 mmol), and stirred at room temperature for one day. The solvent was evaporated under reduced pressure. Separation by column chromatography (silica gel column, developing solvent is dichloromethane). A dark red sticky substance was obtained, which was recrystallized in methanol to obtain 650 mg of a purple solid. Yield 8.9%.
[0061] (2) compound 6 Synthesis
[0062] In a 250 mL two-necked flask, ice-bathed and protected by argon, add 10 mL DMF and 10 mL phosphorus oxychloride. Stir at room temperature for 30 min. Add 100 mL dichloroethane, 5 (595 mg, 1.5 mmol), stirred at 50 °C for 3 h. The solvent was removed under reduc...
Embodiment 3
[0067] Example 3 Fluorescent Probe Compounds 12 Synthesis:
[0068]
[0069] (3) compound 9 Synthesis
[0070] Add 2,4-dimethylpyrrole (3 ml, 30 mmol) and 4-chlorobenzoyl chloride (3.2 g, 18 mmol) into a 100 mL two-necked flask, and stir at room temperature for three days. Add triethylamine (13 mL, 90 mmol), BF 3 ×Et 2 O (15 mL, 120 mmol), and stirred at room temperature for one day. The solvent was evaporated under reduced pressure. Separation by column chromatography (silica gel column, developing solvent is dichloromethane). A deep red viscous substance was obtained, which was recrystallized in methanol to obtain 720 mg of a purple solid. Yield 11%.
[0071] (2) compound 10 Synthesis
[0072] In a 250 mL two-necked flask, ice-bathed and protected by argon, add 10 mL DMF and 10 mL phosphorus oxychloride. Stir at room temperature for 30 min. Add 100 mL dichloroethane, 9 (540 mg, 1.5 mmol), stirred at 50 °C for 3 h. The solvent was removed under reduced...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com