Acellular dermal matrix and preparation method and application thereof
A decellularized dermis and matrix technology, applied in the field of medical biomaterials, can solve the problems that the acellular dermal matrix is not suitable for corneal repair materials, the removal of intracellular substances is not complete, and the preparation method is not described in detail.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
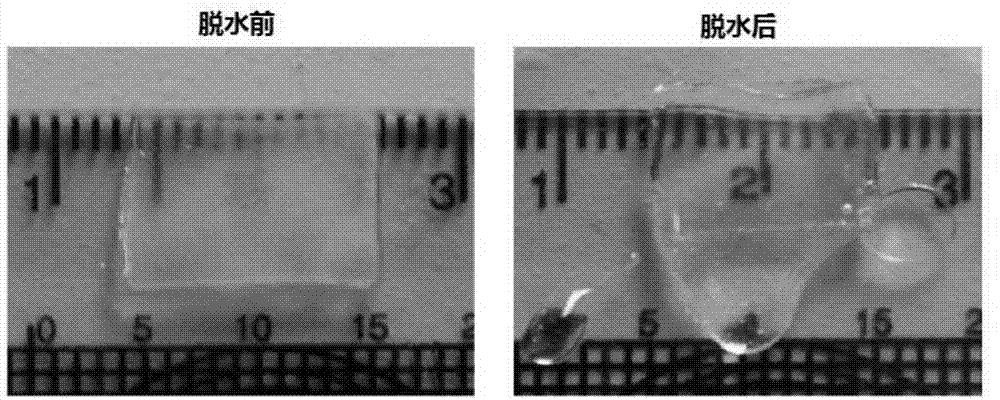
[0051] Example 1 Preparation of acellular dermal matrix
[0052] 1) Obtain the split skin including the epidermis and dermis from the donated corpse; the corpse is purchased from Peking University Health Science Center;
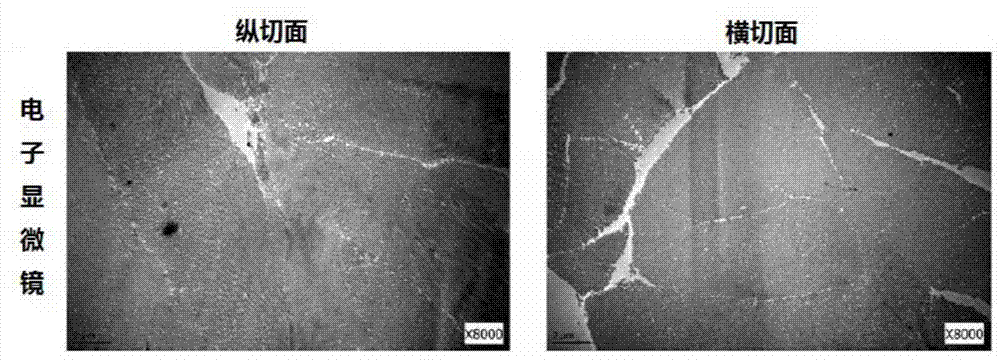
[0053] 2) Processing the cut skin obtained in step 1) into a freeze-dried sheet at a temperature of -15 to -80°C, then removing the 50-200 μm epidermal layer to expose the papillary layer below, and cutting the 800 μm papillary layer to obtain a dermal matrix;
[0054] 3) Use 0.01M sodium phosphate buffer fixative containing 1.5g / 100ml glutaraldehyde to fix the dermal matrix obtained in step 2), the fixation time is 2 hours, and wash with distilled water after fixing;
[0055] 4) using NaOH with an equivalent concentration of 1.5N to remove cellular components in the dermal matrix, and the reaction time is 10 hours to obtain an acellular dermal matrix;
[0056] 5) washing the acellular dermal matrix with 0.01M sodium phosphate aqueous solution for 10 minut...
Embodiment 2
[0059] Example 2 Preparation and use of acellular dermal matrix
[0060] 1) Obtain the split skin including the epidermis and dermis from the corpse;
[0061] 2) Use 0.01M sodium phosphate buffer solution containing 1.5g / 100ml glutaraldehyde to fix the sectioned skin obtained in step 1) for 2 hours, and wash with distilled water after fixing;
[0062] 3) Use KOH alkaline solution with an equivalent concentration of 2.0N to remove the cellular components in the skin, and the reaction time is 60 hours to obtain decellularized split skin;
[0063] 4) washing the decellularized skin with distilled water for 50 minutes;
[0064] 5) Process the decellularized skin section obtained in step 4) into a freeze-dried sheet at a temperature of -27°C, then remove the 50-200 μm epidermal layer to expose the underlying papillary layer, and cut the 100 μm papillary layer to obtain an acellular dermal matrix ;
[0065] 6) Incubate the acellular dermal matrix obtained in step 5) in a 0.1M g...
Embodiment 3
[0068] Example 3 Corneal Transplantation Test
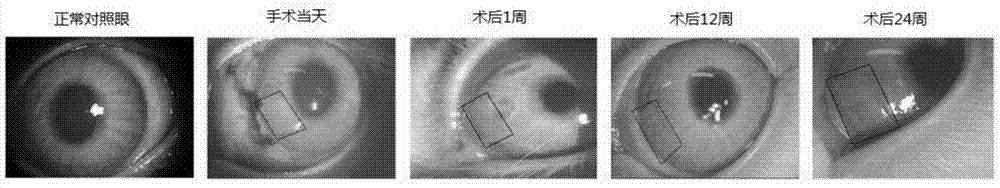
[0069] The acellular dermal matrix with a size of 1 cm×1 cm prepared in Example 1 was cut into small pieces of 2 mm×5 mm, and implanted into the right corneas of 15 New Zealand white rabbits to evaluate the biocompatibility and transparency of the carrier. Under general anesthesia, a 3×6 mm corneal stroma incision was made in the temporal peripheral region along the superior-inferior direction with a depth of half the thickness of the cornea. The ADM was inserted into this incision with forceps without suturing. The unoperated fellow eye served as a positive control. Gentamicin was injected subconjunctivally on the day of surgery. Tobramycin and dexamethasone eye drops were used for the first week after surgery. Subsequent clinical evaluation of corneal light permeability, neovascularization, and rejection was performed through slit lamp examination.
[0070] experiment such as image 3 Shown: The observation period was 24...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com