Method for preparing nano barium strontium titanate/magnesium oxide complex-phase powder in situ by coprecipitation
An in-situ preparation and co-precipitation technology, applied in the direction of magnesium oxide, titanate, nanotechnology, etc., can solve the problems of low interface bonding strength, large extrinsic loss, uneven distribution, etc., and achieve low cost and high particle size The effect of uniformity and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 1) Dissolve a portion of 0.05mol of barium nitrate, 0.05mol of strontium nitrate and 0.1mol of magnesium nitrate in 200ml of deionized water, stir well to obtain a solution containing Ba, Sr and Mg;
[0024] 2) Dissolve 0.2mol oxalic acid in 200ml ethanol and stir evenly to obtain an oxalic acid-ethanol solution;
[0025] 3) Dissolve a portion of 0.1mol butyl titanate in the above oxalic acid-ethanol solution and stir evenly to obtain a titanium oxalate solution;
[0026] 4) Mix the solution containing Ba, Sr, Mg and the solution containing Ti obtained above, stir evenly, and then divide into six solutions;
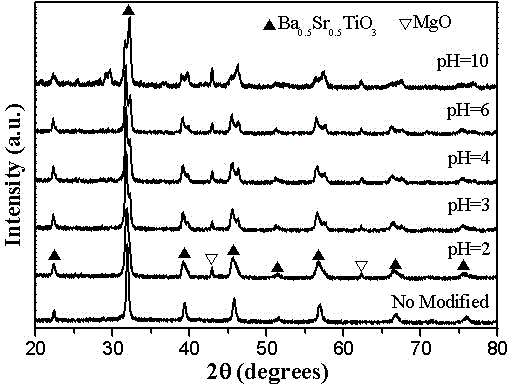
[0027] 5) Slowly add ammonia water to the five solutions to adjust the pH value, and obtain five kinds of pH=2, pH=3, pH=4, pH=6 and pH=10 (1- x ) Ba 0.5 Sr 0.5 TiO 3 - x MgO( x =0.5) precursor solution;
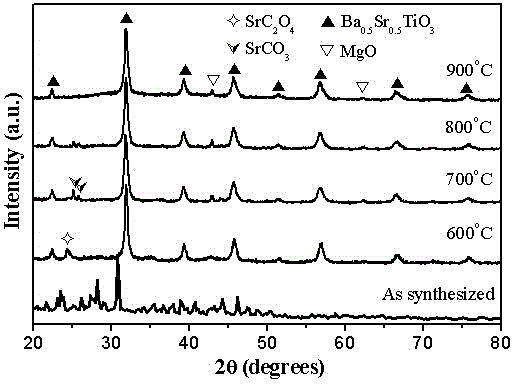
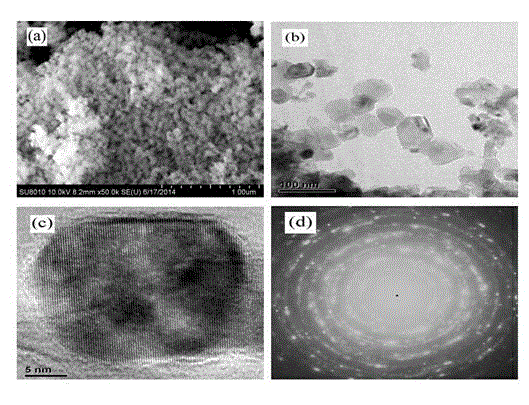
[0028] 6) The above six (1- x ) Ba 0.5 Sr 0.5 TiO 3 - x MgO( x =0.5) The precursor solution was subjected to co-precipitation reaction for 48h, then...
Embodiment 2
[0033] 1) Mix a part of 0.0475mol of barium nitrate, 0.0475mol of strontium nitrate and 0.005mol of magnesium nitrate, a part of 0.035mol of barium nitrate, 0.035mol of strontium nitrate and 0.03mol of magnesium nitrate, a part of 0.025mol of barium nitrate , 0.025mol of strontium nitrate and 0.05mol of magnesium nitrate, a portion of 0.005mol of barium nitrate, 0.005mol of strontium nitrate and 0.09mol of magnesium nitrate, respectively dissolved in 100ml of deionized water, stirred evenly to obtain four and a solution of Mg;
[0034] 2) Dissolve one part of 0.19mol oxalic acid, one part of 0.14mol oxalic acid, one part of 0.10mol oxalic acid and one part of 0.02mol oxalic acid in 100ml ethanol and stir well to obtain four oxalic acid-ethanol solutions;
[0035] 3) Dissolve one part of 0.095mol butyl titanate, one part of 0.07mol butyl titanate, one part of 0.05mol butyl titanate and one part of 0.01mol butyl titanate in the above oxalic acid-ethanol solution and stir well, ...
Embodiment 3
[0042] 1) Mix one part of 0.019mol of barium nitrate, 0.076mol of strontium nitrate and 0.005mol of magnesium nitrate, one part of 0.014mol of barium nitrate, 0.056mol of strontium nitrate and 0.03mol of magnesium nitrate, one part of 0.01mol of barium nitrate , 0.04mol of strontium nitrate and 0.05mol of magnesium nitrate, a portion of 0.002mol of barium nitrate, 0.008mol of strontium nitrate and 0.09mol of magnesium nitrate, respectively dissolved in 100ml of deionized water, stirred evenly to obtain four and a solution of Mg;
[0043] 2) Dissolve one part of 0.19mol oxalic acid, one part of 0.14mol oxalic acid, one part of 0.10mol oxalic acid and one part of 0.02mol oxalic acid in 100ml ethanol and stir well to obtain four oxalic acid-ethanol solutions;
[0044] 3) Dissolve one part of 0.095mol butyl titanate, one part of 0.07mol butyl titanate, one part of 0.05mol butyl titanate and one part of 0.01mol butyl titanate in the above oxalic acid-ethanol solution and stir well,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com