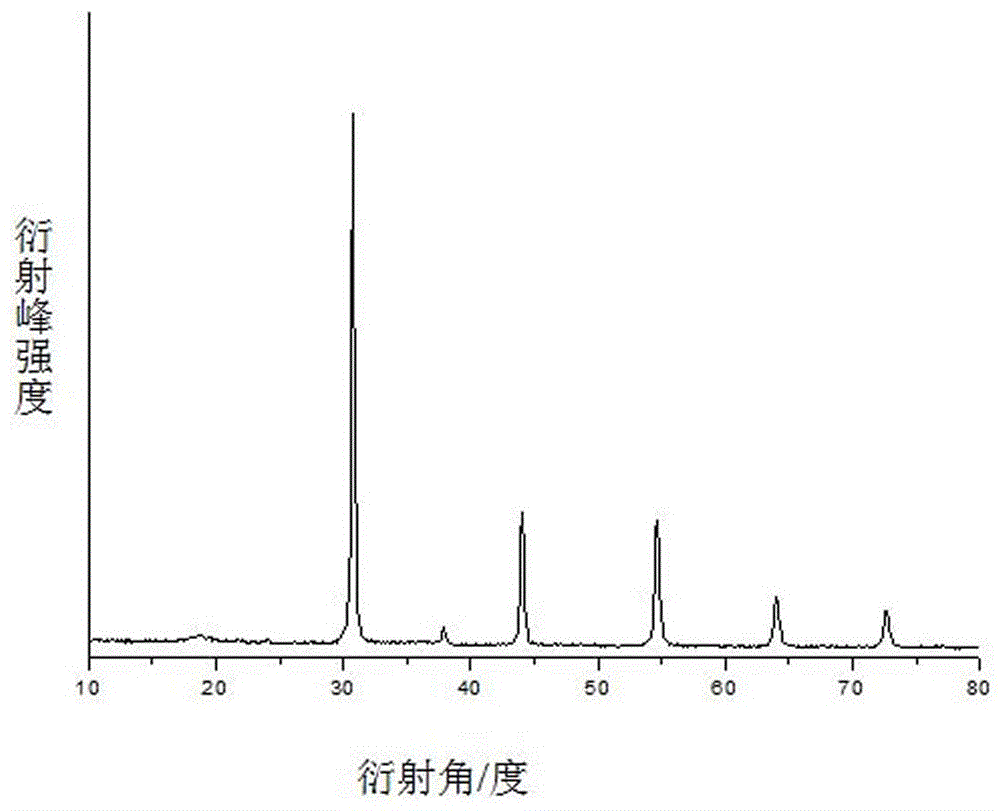
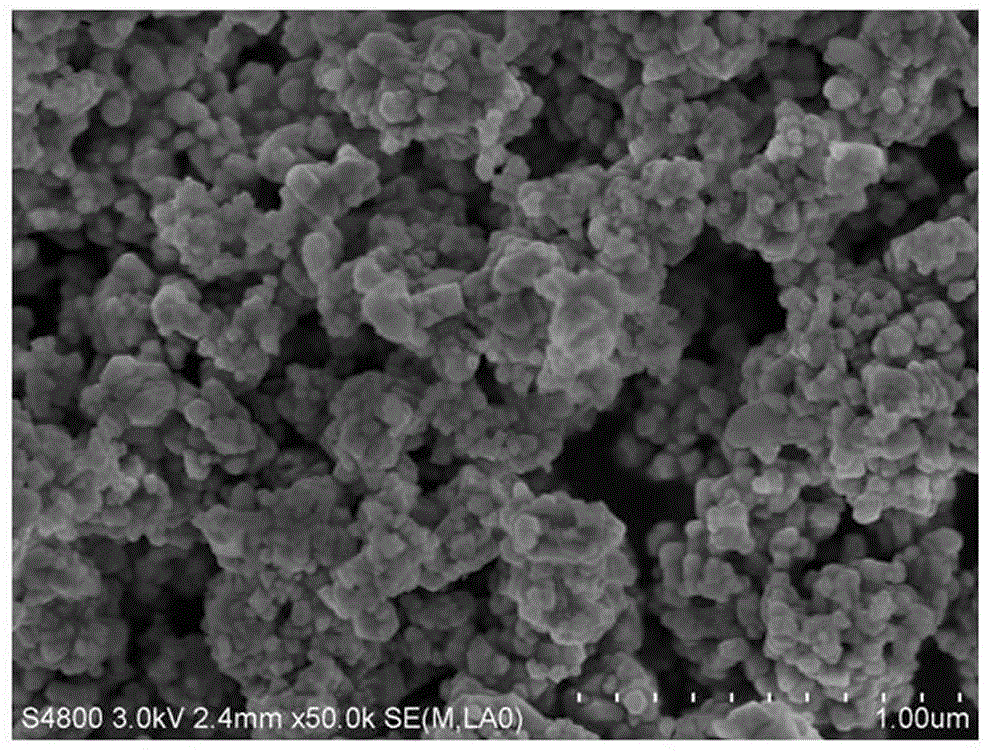
A kind of preparation method of barium scandium tantalate powder
A technology for barium scandium tantalate and powder, which is applied in the field of preparation of barium scandium tantalate powder, can solve the problems of restricting the production of barium scandium tantalate, taking a long time, etc., and achieves obvious energy saving effect, low preparation cost, impurity low content effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Accurately weigh barium nitrate, tantalum pentachloride, scandium nitrate and citric acid, put them into methanol aqueous solution (volume ratio 1:1) and stir to dissolve. The solution was subjected to cross-linking reaction at 500°C for 6 h. After the reaction, it was naturally cooled to room temperature, and molten salt (the molten salt was a mixture of sodium chloride and barium chloride, the molar ratio of sodium chloride and barium chloride was 1: 1) After mixing, put it into a mortar and grind it finely for 30 minutes. The molar ratio of the barium nitrate, tantalum pentachloride, scandium nitrate, molten salt and citric acid is 2:1:1:4:60. Put the above-mentioned mixture into a crucible and put it into a box-type resistance furnace for calcination reaction. The reaction temperature is 900°C and the reaction time is 8 h. After cooling, it is washed with water and dried to prepare scandium barium tantalate powder. The purity of its products is not less than 99.82%...
Embodiment 2
[0037] Accurately weigh barium nitrate, tantalum pentaethoxide, scandium nitrate and urea, put them into aqueous methanol solution (volume ratio 1:1) and stir to dissolve. The solution was subjected to cross-linking reaction at 150°C for 10 h. After the reaction, it was naturally cooled to room temperature, and molten salt (the molten salt was a mixture of sodium chloride and barium chloride, the molar ratio of sodium chloride and barium chloride was 1: 1) After mixing, put it into a mortar and grind it finely for 30 minutes. The molar ratio of the barium nitrate, tantalum pentaethoxide, scandium nitrate, molten salt and urea is 2:1:1:4:100. Put the above mixture into a crucible and put it into a box-type resistance furnace for calcination reaction at a reaction temperature of 700°C and a reaction time of 24 h. After cooling, wash and dry it with water to prepare scandium barium tantalate powder. The purity of its products is not less than 99.89%, and the impurity content: ca...
Embodiment 3
[0039]Accurately weigh barium nitrate, tantalum pentachloride, scandium nitrate and citric acid, put them into methanol aqueous solution (volume ratio 1:1) and stir to dissolve. The solution was subjected to cross-linking reaction at 450°C for 8 h. After the reaction, it was naturally cooled to room temperature, and molten salt (the molten salt was a mixture of sodium chloride and barium chloride, the molar ratio of sodium chloride and barium chloride was 1:1) ) after mixing, put it into a mortar and grind it finely, and the grinding time is 30 minutes. The molar ratio of the barium nitrate, tantalum pentachloride, scandium nitrate, molten salt and citric acid is 2:1:1:4:60. Put the above mixture into a crucible and put it into a box-type resistance furnace for calcination reaction. The reaction temperature is 1000°C and the reaction time is 6 h. After cooling, it is washed with water and dried to prepare scandium barium tantalate powder. The purity of its products is not les...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com