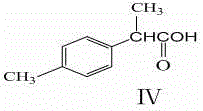
2-(4-methylphenyl)propionic acid syntehsis method
A technology of methyl phenyl and synthetic methods, which is applied in the direction of chemical instruments and methods, carboxylate preparation, carboxynitrile preparation, etc., can solve the problems of many types of chemical raw materials, many organic solvents, and large amounts of waste water, and achieve easy Realize the effects of industrialization, stable reaction and short process route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1: A kind of synthetic method of 2-(4-methylphenyl) propionic acid, comprises the following steps:
[0056] (1) Chlorination reaction: the preparation of p-methylbenzyl chloride (compound Ⅰ), the specific operation steps are as follows:
[0057] (1) Add 250L of p-xylene liquid into the reactor, fill the tail gas suction tank of the reactor with water, and the water level is 40-70%. Turn on the reaction kettle to stir, heat the p-xylene to about 75°C and keep it at a constant temperature, turn on a 100W blue light with a wavelength of 450nm to irradiate, and slowly introduce chlorine gas under light conditions to react (flow rate 5-10m 3 / hr), and follow-up analysis to monitor the product situation.
[0058] (2) Analysis results When p-methyl benzyl chloride is about 30% and other chlorides are detected, such as p-dimethyl chlorobenzene, p-dichloro benzyl or polychloride, close the chlorine gas valve and stop heating , turn off the light source and stop the ...
Embodiment 2
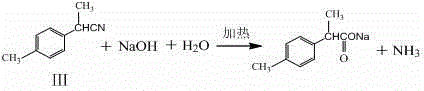
[0090] Example 2: A kind of synthetic method of 2-(4-methylphenyl)propionic acid, the specific operation steps of step (1), step (2) and step (4) are the same as those in Example 1, the difference lies in step (3) Methylation reaction: the preparation of 2-(4-methylphenyl)propionitrile (compound Ⅲ), the specific operation steps are as follows:
[0091] (1) Add 30kg (0.229kmol) of p-tolueneacetonitrile and 150kg (1.665kmol) of dimethyl carbonate into the reactor respectively from the feeding product, and then add 2.1kg of triethanolamine from the feeding port of the basic substance.
[0092] (2) Pressure test: Use nitrogen to test the pressure, the pressure is about 3Mpa, and there is no leakage. Slowly release pressure and discharge nitrogen. Close the valve.
[0093] (3) Turn on stirring and set the stirring speed.
[0094] (4) Turn on the exhaust gas cooler to circulate cooling water.
[0095] (5) Turn on the reactor heating device. Gradually raise the temperature and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com