Recombinant human interferon beta-1b freeze-dried preparation and preparing method thereof
A technology for recombinant human interferon and freeze-dried preparations, which is applied in peptide preparation methods, freeze-dry delivery, chemical instruments and methods, etc., and can solve the risk of increasing solvent residues, increasing interferon binding capacity, and increasing the risk of adverse reactions, etc. problems, to achieve the effect of reducing the risk of adverse reactions, good stability and active characteristics, and product safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Embodiment 1: preparation stock solution
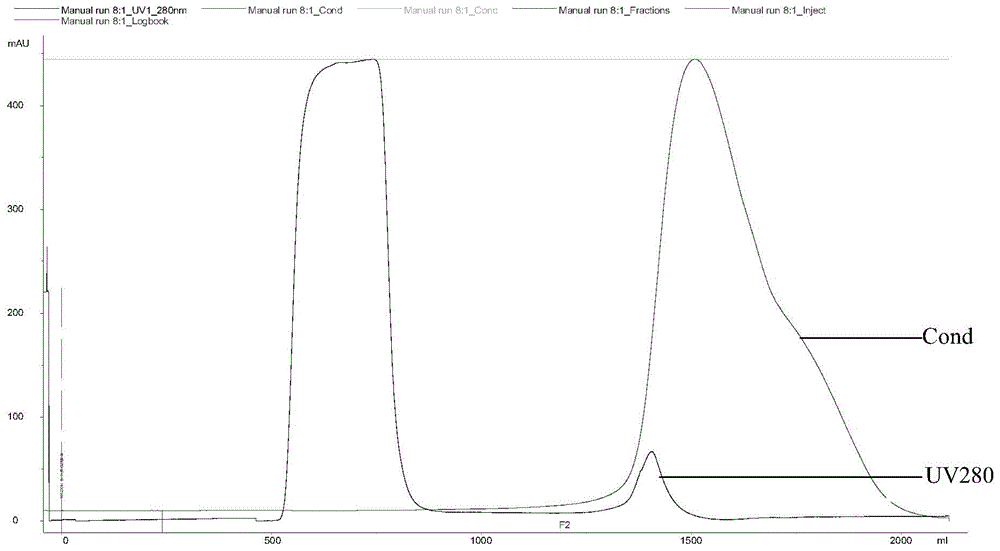
[0056] SEC chromatographic desalting preparation stock solution
[0057] The Escherichia coli fermentation broth is collected and purified to obtain a recombinant human beta-1b interferon solution. The solution system at this time is 0.1% (w / v) SDS + 10-100mM phosphate buffer solution, pH 7.0-7.4, so it is necessary to replace the interferon buffer system with the original preparation solution system by buffer replacement, and at the same time Residues of related substances in the original system were reduced to below the level specified in the Pharmacopoeia. The medium was changed by SEC chromatographic desalting method. Sephadex G-25Superfine was selected for SEC chromatographic desalting, size: 5.0×40cm, flow rate: 10mL / min, and mobile phase was 2.5Mm NaOH with pH 11.5. The samples were collected from the peak 5mAu to the peak tail 5mAu protein from UV280. The collected samples were mixed evenly and sterilized by filtrat...
Embodiment 2
[0061] Embodiment 2: acridine orange-spectrophotometric detection of SDS residues
[0062] After mixing the SDS standard substance with acridine orange at different concentrations, extract it with toluene, then measure the absorbance value of the toluene extraction phase at 499nm of visible light to obtain a standard curve, and calculate its SDS concentration.
[0063] Precisely prepare 10ml of SDS solutions with concentrations of 0, 0.005, 0.01, 0.02, 0.03, and 0.04g / L respectively. 0.5mol / L NaHSO 4 50ml of 0.4% acridine orange solution for solution configuration.
[0064] Take 100ul of SDS solutions of different concentrations and the sample to be tested in a stoppered test tube, and add 100ul of 0.5mol / L NaHSO 4 Mix the 0.4mol / L acridine orange solution prepared by the solution. After adding 3ml of toluene, plug the test tube tightly, shake vigorously for 3min, centrifuge at 2000g / min for 5min, take the supernatant and measure the absorbance at 499nm.
[0065] Table 1 ...
Embodiment 3
[0073] Embodiment 3: Semi-finished product preparation sub-package
[0074] The stock solution system of the interferon preparation obtained after desalting is a sodium hydroxide system, and the interferon can only maintain a stable activity in a short period of time, and this system cannot be used as the stock solution for testing, so the buffer system needs to be replaced again. Add stabilizers and buffers according to the following prescription composition and final concentration, and make semi-finished products after settling to a certain volume:
[0075] Recombinant human interferon β-1b 0.1~0.5mg / ml
[0076] Phosphate buffer 10~30mM
[0077] Human serum albumin (HSA) 1-3%
[0078] HSA within this concentration range is suitable for maintaining the solubility and activity stability of the recombinant human interferon-β-1b dosage form after freeze-drying; while the phosphate buffer is more conducive to maintaining the dissolved state and activity of this protein. During...
PUM
| Property | Measurement | Unit |
|---|---|---|
| correlation coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com