Plasmid for detecting activity of ribosome inactivating protein as well as construction method and application of plasmid
A technology for ribosome inactivation and protein activity, applied in the field of genetic engineering, can solve the problems of high cost, complicated steps, low sensitivity, etc., and achieve the effect of reducing cost, large application potential, and solving low sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: psiCHECK TM - Construction of 2-F28RNA plasmid
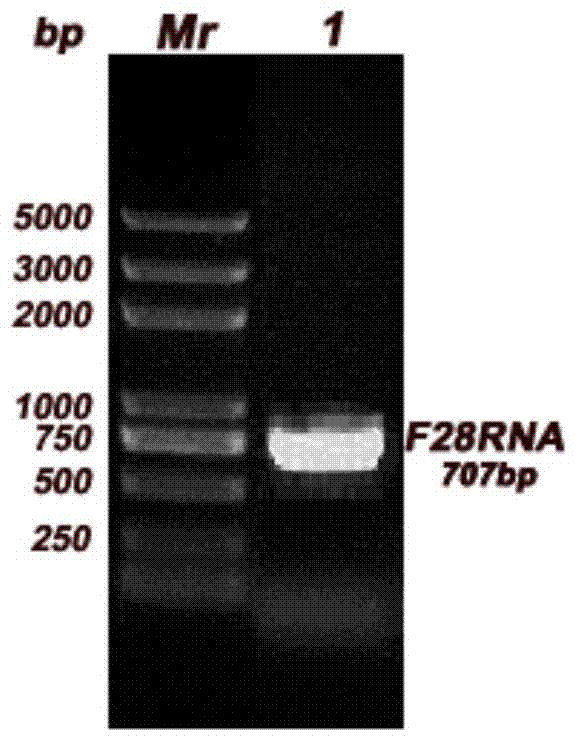
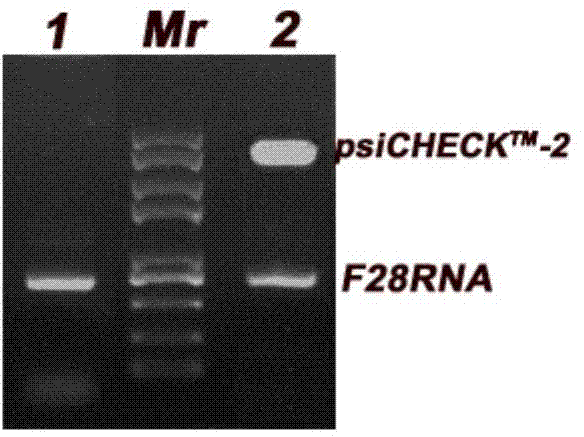
[0025] According to the human 28S ribosomal sequence reported in GenBank as a template, primers were designed respectively upstream and downstream of the active site CGAGAG, F28F: CTCGAG AGCTCGCTTGATCTTGATTTTCA,F28R: CGCCGGCG TCTACGAATGGTTTAGCGCCA introduced Xho I and Not I restriction sites at the 5' and 3' ends respectively; the RNA of HCT116 cells was extracted by the Trizol method; the gene fragment F28RNA ( figure 1 ); the obtained gene fragment F28RNA and psiCHECK TM -2 plasmid was digested with restriction endonucleases Xho I and Not I, separated by 1% agarose gel electrophoresis, and the gene fragment F28RNA and psiCHECK were recovered TM -2 vector; use DNA ligase to convert the recovered gene fragment F28RNA and psiCHECK TM -2 vector for ligation, and the ligated product was transformed into Escherichia coli DH5α, after screening positive clones ( figure 2 ), a large amount of amplification was...
Embodiment 2
[0026] Example 2: Detection of ribosome inactivating protein activity in vitro
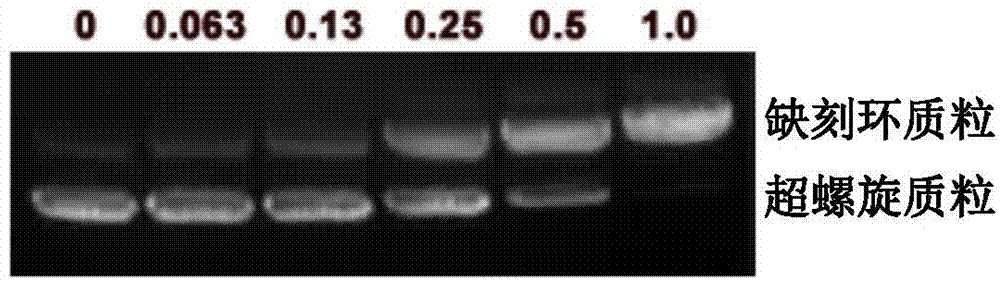
[0027] will psiCHECK TM- 1 μg of 2-F28RNA plasmid was dissolved in 10 μL of PBS buffer (pH 7), and buckwheat ribosome inactivating protein in different contents was added, incubated at 37° C. for 30 minutes, and detected by agarose gel electrophoresis. Ribosome inactivating protein activity was characterized by the amount of plasmid state transition from supercoiled to nicked loop (see image 3 ).
Embodiment 3
[0028] Example 3: Intracellular Detection of Ribosome Inactivation Protein Activity Detection
[0029] will psiCHECK TM - Dissolve 4 μg of 2-F28RNA plasmid in 400 μL of serum-free medium, add 8 μL of transfection reagent (TurboFect Transfection Reagent), and incubate at room temperature for 15 minutes; add the above mixed reagent to the cell culture well to be transfected in the cell culture medium, The cell abundance should reach more than 95%, and continue to culture at 37°C for about 24 hours; digest the transfected cells with trypsin, and distribute equal amounts into 3 wells, of which 1 μg and 5 μg are added to well 1 and 2 respectively For buckwheat ribosome inactivating protein, add an equal amount of PBS to the No. 3 well as a blank control group, and incubate at 37°C for about 12 hours; collect cells, lyse the cells with cell lysate, centrifuge to get the supernatant; take 20 μL of cell lysate, add to measure Add 100 μL firefly luciferase detection reagent to the bot...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com