Synthetic method for 5-acetylsalicylamide
A technology of acetylsalicylic amide and synthesis method, which is applied in the preparation of carboxylic acid amides, chemical instruments and methods, preparation of organic compounds, etc. The effect of short time, high yield and high product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0039] Embodiment 1-1: a kind of synthetic method of 5-acetyl salicylamide, carry out following steps successively:
[0040] 1), NaCl-AlCl 3 Preparation of low melting point mixed molten salt system:
[0041] Place a 100ml three-neck flask, a mechanical stirrer, a condenser (linked to the tail gas HCl absorption device), a 0-200°C thermometer, and a constant pressure dropping funnel in a constant temperature oil bath; quickly weigh 0.0648mol (about 8.64g ) anhydrous aluminum chloride and 0.0648mol (about 3.79g) sodium chloride were added into the flask, mechanical stirring was started, and the temperature was raised to 140°C until the solid in the system melted and the temperature stabilized.
[0042] Remarks: After about 25 minutes, both anhydrous aluminum chloride and sodium chloride are in a molten state.
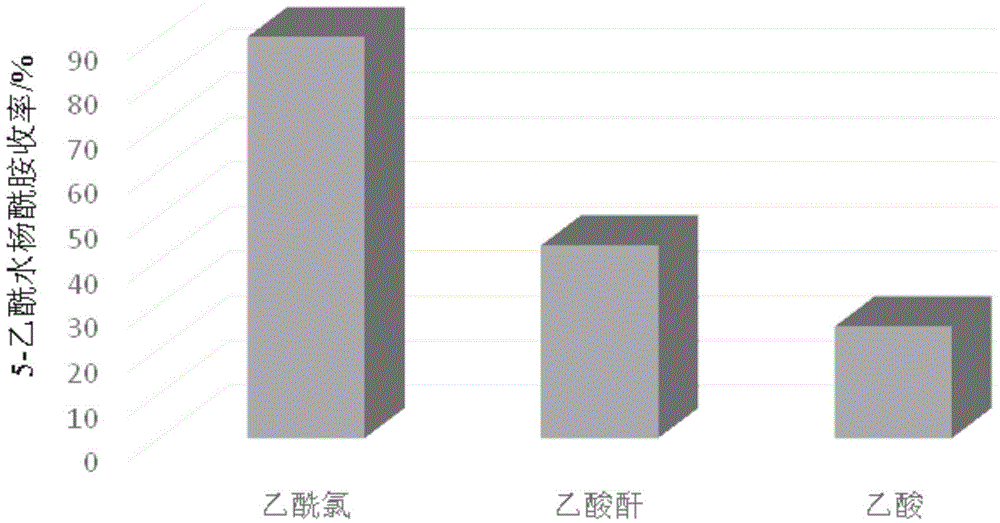
[0043] 2) Weigh 0.036 mol (about 5.00 g) of salicylamide into the above-mentioned flask (the flask is kept at 140° C.) under stirring conditions, and melt it until the...
Embodiment 1-2
[0049] Example 1-2, the equivalent of anhydrous aluminum chloride remains unchanged, and the "anhydrous aluminum chloride: sodium chloride = 1:1 molar ratio" in Example 1-1 is changed to: anhydrous chlorination Aluminum: sodium chloride = 2:1, 3:2, 1:2, 1:3; and adjust the corresponding temperature according to the stability of the system melt, as follows:
[0050] When the mol ratio of aluminum chloride anhydrous: sodium chloride=2:1, the temperature of step 1)~step 3) is all changed to 167 ℃;
[0051] When the mol ratio of aluminum chloride anhydrous: sodium chloride=3:2, the temperature of step 1)~step 3) is all changed to 125 ℃;
[0052] When the molar ratio of anhydrous aluminum chloride:sodium chloride=1:2, the temperature of step 1)~step 3) still keeps 140 ℃;
[0053] When the molar ratio of anhydrous aluminum chloride:sodium chloride=1:3, the temperature of step 1) to step 3) is still kept at 140°C.
Embodiment 1
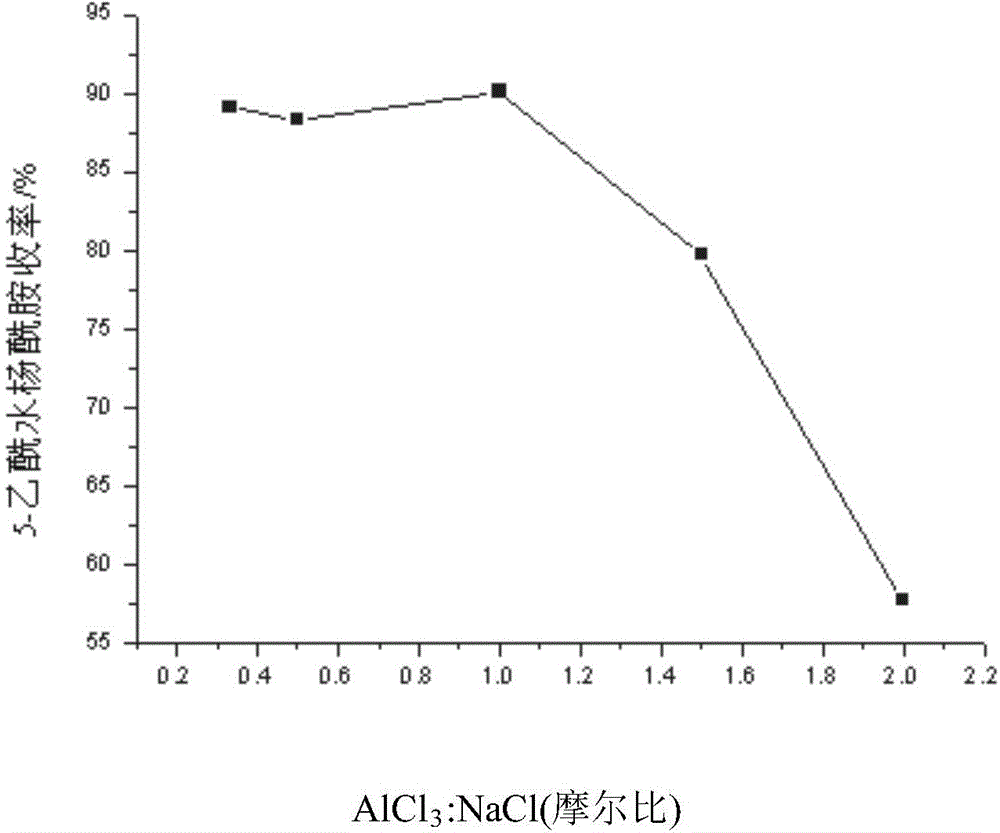
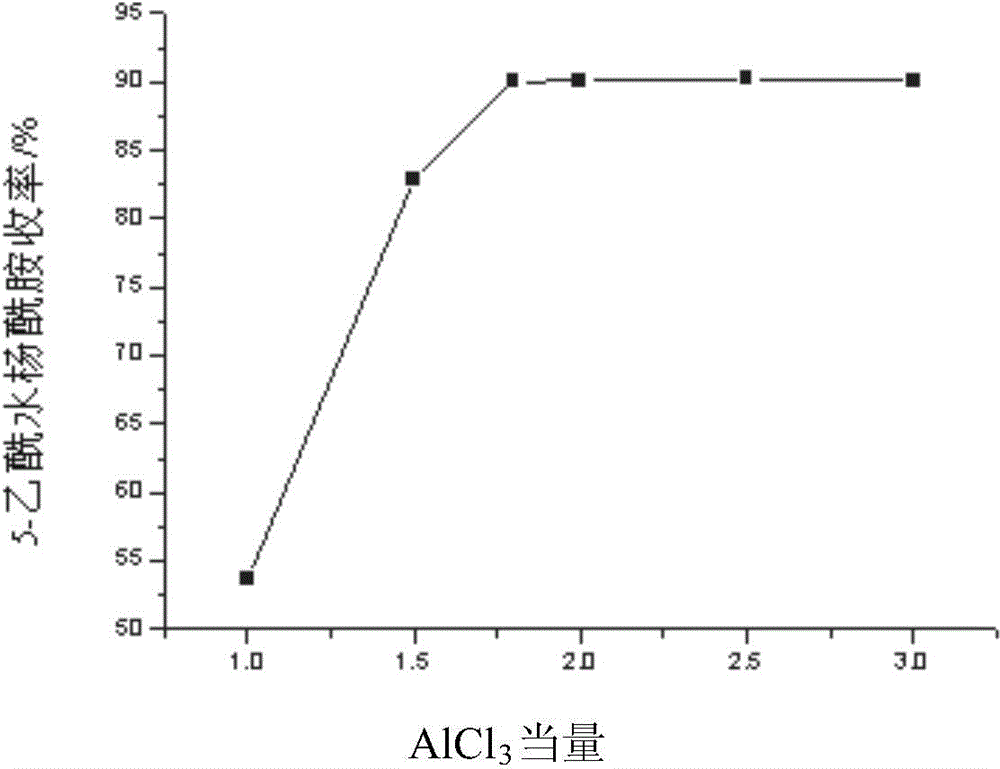
[0056] Embodiment 1-3, keeping NaCl-AlCl 3 The molar ratio of aluminum chloride and sodium chloride in the low melting point mixed molten salt system is 1:1, and the equivalents of aluminum chloride in Example 1 are respectively changed to 1.0eq, 1.5eq, 2.0eq, 2.5eq, 3.0eq, All the other contents are equal to embodiment 1-1; The yield comparison of the 5-acetyl salicylamide of final gain figure 2 shown.
[0057] When the aluminum chloride equivalent is less than 1.8eq, the raw material cannot be completely converted, especially when the equivalent is 1.0eq, because the solvent is too little, the reaction system is easily coked to generate a large amount of by-products, so the product yield is low. And when the aluminum chloride equivalent is greater than 1.8eq, the catalyst amount is excessive, and the excess molten salt only exists as a solvent, and it is unnecessary to increase the equivalent of anhydrous aluminum chloride. Therefore, the aluminum chloride equivalent is o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com