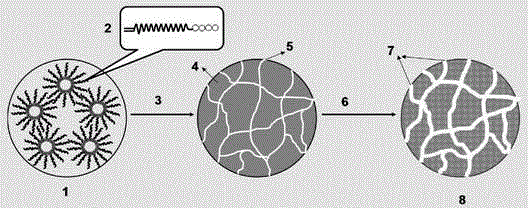
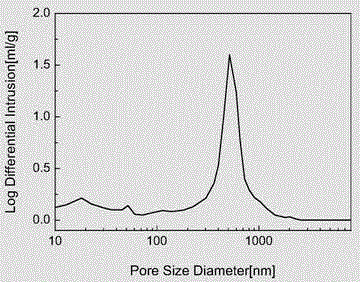
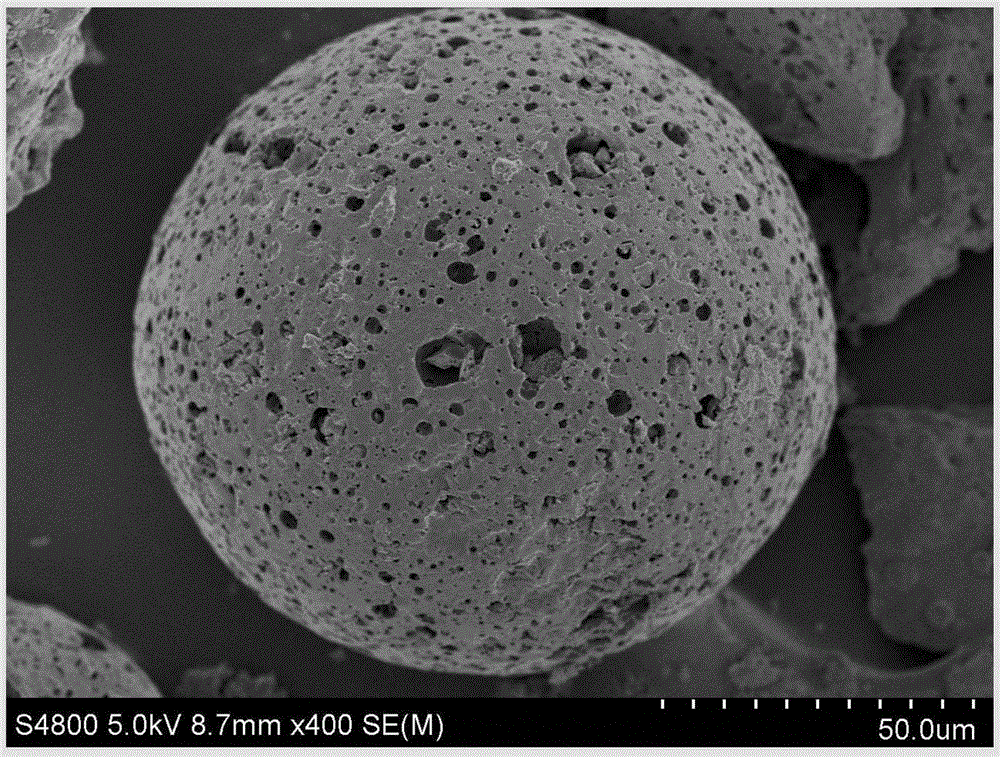
Hydrophilic super-macroporous polymer microsphere and preparation method thereof
A polymerization method and linear polymer technology, applied in the field of hydrophilic ultra-macroporous polymer microspheres and their preparation, can solve the problems of poor strength of microspheres
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] 1) Synthesis of poly-3-O-methacryloyl-diacetone-D-galactose (PMDAGal) by ATRP reaction
[0059] Add a stir bar to the Schlenk bottle at room temperature, then add cuprous bromide (6.45mg), N,N,N,N',N'-pentamethyldiethylenetriamine (PMDETA) (9.0mg) in sequence ), 3-O-methacryloyl-diacetone-D-galactose (MDAGal) (1.52g) and 4ml of toluene. After three cycles of liquid nitrogen freezing-pumping-charging-thawing, oxygen is removed. Finally, the initiator 1-bromoethylbenzene (18.3mg) was added and reacted at 60°C for 4 hours. The reaction product was dissolved with chloroform, and the reaction product was diluted and passed through an alumina column to remove the catalyst. The colorless solution obtained was precipitated twice with methanol, filtered under suction at room temperature, and dried in vacuum to obtain 0.79 g of PMDAGal. The molecular weight Mn=6835 was determined by gel permeation chromatography (GPC).
[0060] 2) Synthesis of poly3-O-methacryloyl-diacetone-D-galacto...
Embodiment 2
[0068] 1) Synthesis of PMDAGlu using ATRP reaction
[0069] Add a stir bar to the Schlenk bottle at room temperature, and then add cuprous chloride (3.2mg), PMDETA (7.1mg), MDAGlu (0.98g) and 3ml chlorobenzene in sequence. After three liquid nitrogen freezing-pumping-aeration -Defrost cycle process to remove oxygen. Finally, add the initiator ethyl 2-bromoisobutyrate (30.4mg), react at 50°C for 4h, dissolve the reaction product with tetrahydrofuran, dilute the reaction product and pass through an alumina column to remove the catalyst. The colorless solution obtained was precipitated twice with methanol, filtered under suction at room temperature, and dried in vacuum to obtain 0.59 g of PMDAGlu. The molecular weight Mn=4631 was detected by gel permeation chromatography (GPC).
[0070] 2) Synthesis of PMDAGlu-PS by ATRP reaction
[0071] Add a stir bar to the Schlenk bottle at room temperature, then add cuprous bromide (14.6mg), PMDETA (22.8mg), styrene (2.43g) in sequence, and go th...
Embodiment 3
[0078] 1) Synthesis of poly-3-O-methallyl-diacetone-D-glucose (PMAlDAGlu) by AGET-ATRP reaction
[0079] In a 100ml three-necked flask with a stopper at room temperature, add 4ml of rysulfen, MAlDAGlu (1.42g), FeCl3 (8.4mg), triphenylphosphine (35.4mg) and methyl 2-bromopropionate (75.3mg) in sequence, and stir Make it evenly mixed, deoxidize with nitrogen for 10 minutes, add the reducing agent ascorbic acid (7.9mg), react at 55°C for 2h under nitrogen protection, dissolve the reaction product with tetrahydrofuran, precipitate twice with methanol, filter with suction at room temperature, and dry in vacuum , Obtain 0.49gPMDAGal, the molecular weight Mn=1356 is detected by gel permeation chromatography (GPC).
[0080] 2) Synthesis of poly-3-O-methallyl-diacetone-D-glucose-polystyrene block copolymer (PMAlDAGlu-PS) by AGET-ATRP reaction
[0081] Add styrene (2.36g) and FeCl into a 100ml three-neck bottle with stopper at room temperature 3 (10.8mg), triphenylphosphine (44.96mg), PMAlDAG...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com