Glucose-sensitive self-regulated insulin release micro-gel carrier and preparation method thereof
A glucose-sensitive, microgel technology, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problem of slow release, automatic "on-off" release of insulin, Low LCST issues, to achieve good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
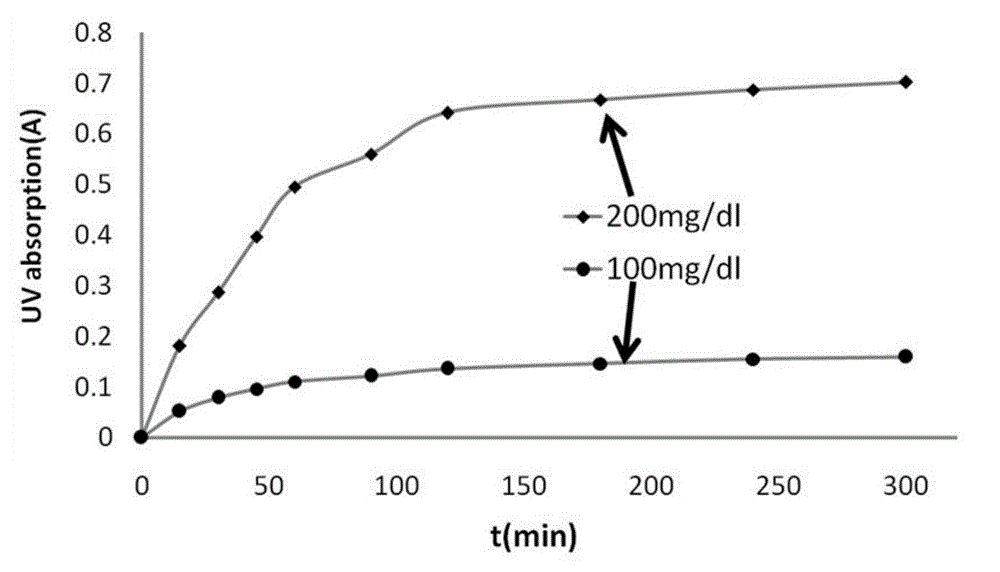
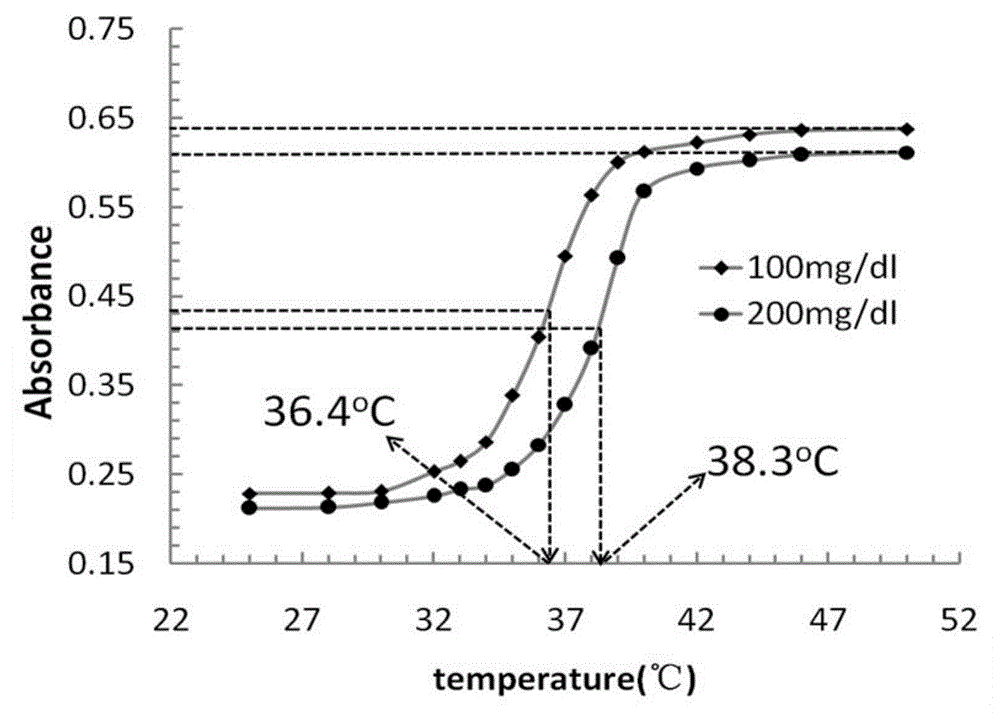
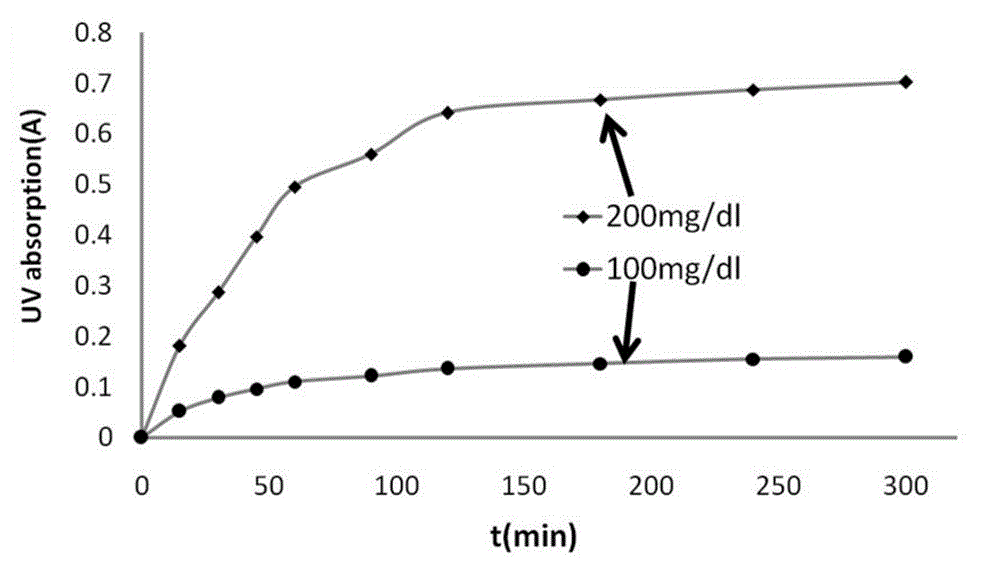
Image
Examples
Embodiment 1
[0044] (1) Synthesis of vinyl functional group RGD polypeptide: Glycine (FMOC-Gly-OH) protected by 9-fluorenylmethoxycarbonyl group from the amino group, carboxyl group on the amino group and side group were respectively protected by 9-fluorenylmethoxycarbonyl group and tert-butyl The protected aspartic acid (FMOC-Asp(OtBu)-OH), the amino group and the guanidine group on the side group were respectively replaced by 9-fluorenylmethoxycarbonyl and 2,2,4,6,7-pentamethyl-2H- Starting from benzofuran-5-sulfonyl-protected arginine (FMOC-Arg(Pbf)-OH), a small molecule polypeptide containing RGD sequence was synthesized by means of peptide solid-phase synthesis, and then reacted with acrylic acid to obtain a vinyl function The RGD sequence small molecule polypeptide with a strong hydrophilic group, the synthesis steps are as follows (see the synthesis schematic diagram figure 1 ).
[0045] Weigh 1.50 g of 2-chlorotrityl chloride resin, put it into a porous filter funnel polypepti...
Embodiment 2
[0070] (1) Synthesis of vinyl functional group RGD polypeptide: see Example 1.
[0071] (2) Synthesis of 3-acrylamidophenylboronic acid: See Example 1.
[0072] (3) Preparation of glucose-sensitive microgel: Weigh 118.4 mg (0.62 mmol, 9.3 mol%) of monomer 3-acrylamidophenylboronic acid, 280 mg (0.7 mmol, 10.5 mol%) of hydrophilic RGD polypeptide containing vinyl functional groups mol%), N - Isopropylacrylamide 565 mg (5 mmol, 74.9 mol%) and cross-linking agent N,N' -Methylenebisacrylamide 32.3 mg (0.21 mmol, 3.1 mol%), and measure 100 mL of distilled water, add it to a three-necked flask equipped with a condensing device, a nitrogen conduit, a constant pressure dropping funnel, and a magnetic stir bar, and open the Stir, blow nitrogen for 30 min, and heat to 60-80 o C, 34.2 mg (0.15 mmol, 2.2 mol%) of the initiator ammonium persulfate (APS) was added through a constant-pressure dropping funnel at a speed of 1000 r / min, and reacted for 24 h under a nitrogen atmosphere to pr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com