Preparation method for sodium zoledronic acid
A technology of sodium zoledronic acid and zoledronic acid, which is applied in the field of drug synthesis, can solve problems such as the residual amount of organic solvents easily exceeding the standard, unfavorable industrial production, and non-compliance with pharmaceutical standards, and achieves easy separation and purification and high yield , post-processing convenient effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
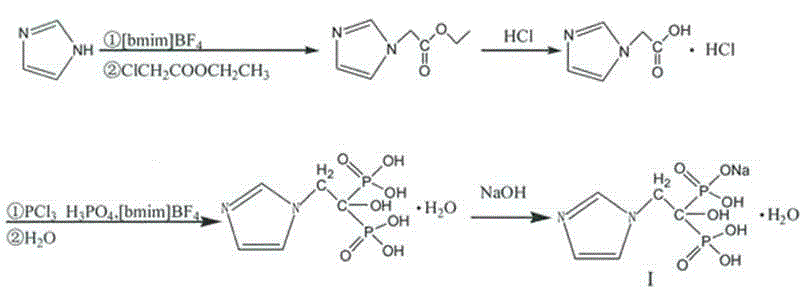
[0025] Synthesis of Sodium Zoledronate
[0026]
[0027] (1) Synthesis of ethyl imidazol-1-yl acetate
[0028] Add imidazole (13.62g, 0.2mol) and [bmim]BF in turn to the three-neck flask 4 (100mL), stir and heat up to 60°C, under reflux, slowly add ethyl chloroacetate (24.51g, 0.2mol) dropwise, the dropwise addition time is about 2h, keep stirring under reflux for 16h, TLC monitors the end point of the reaction and shows There was no raw material point, the reaction was completed, and the temperature was lowered to room temperature to obtain a crude product of ethyl imidazol-1-yl acetate, about 24 g, which was directly used in the next reaction without further purification.
[0029] (2) Synthesis of imidazol-1-ylacetic acid hydrochloride
[0030] Add 24g of the crude ethyl imidazol-1-yl acetate prepared above into a three-necked flask, add concentrated hydrochloric acid (34mL), heat up to 85°C with exotherm, continue to heat up to reflux, stir and react under reflux f...
Embodiment 2
[0036] Influence of Phosphorus Trichloride Adding Time on Reaction Yield
[0037] Fix other conditions unchanged, only change the rate of addition of phosphorus trichloride, the impact of rate of addition on the yield of sodium zoledronic acid is shown in Table 1, and the test results show that when phosphorus trichloride is added dropwise too fast, a large amount of gas will be produced instantaneously , the reaction is very intense, the liquid splashes, and the temperature rises rapidly, resulting in a low yield. If it is added dropwise slowly, the reaction speed is too slow and the consumption time is too long, so 4h is the best dropwise addition time.
[0038] Dropping time (h) Yield (%) 2.5 51.7 3.5 71.6 4.0 90.1 4.5 90.1 5.5 88.5
Embodiment 3
[0040] Effect of Reaction Temperature on the Yield of Sodium Zoledronate
[0041] With other conditions kept constant, the effect of the reaction temperature of the condensation of imidazol-1-ylacetic acid hydrochloride with phosphoric acid and phosphorus trichloride on the yield of zoledronic acid monohydrate, the results are shown in Table 2. The test results show that as the temperature increases, the yield increases gradually, but when the reflux temperature is high, the yield shows a downward trend, which is due to the decomposition of phosphorus trichloride, resulting in a decrease in reactants. In addition, when the temperature is too high, the loss of solvent volatilization and solvent leakage will inevitably increase. When the reflux temperature is low, the reaction rate is slow and the reaction is insufficient, so the yield is low. Therefore, the optimal reaction temperature is about 65 ° C.
[0042] temperature (°C) Yield (%) 55 63.4 60 78.5 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com