Stable compound coenzyme preparation as well as preparation method and applications thereof
A coenzyme and stable technology, applied in the field of pharmaceutical preparations, can solve problems such as unstable batch quality, reduced filtration speed, long centrifugation time, etc., achieve good freeze-drying effect, reduce poor shape formation, and have great reference value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
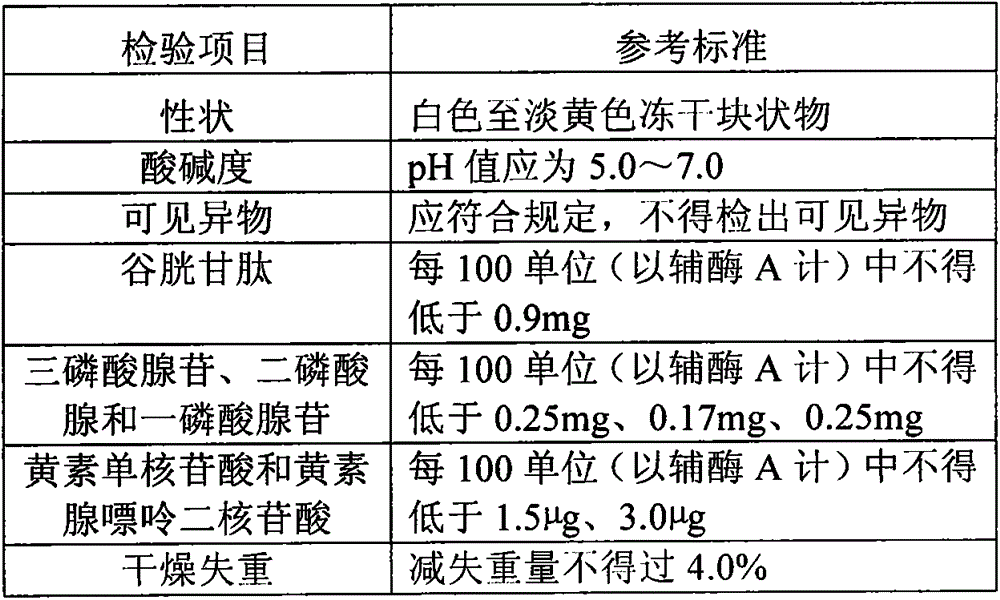
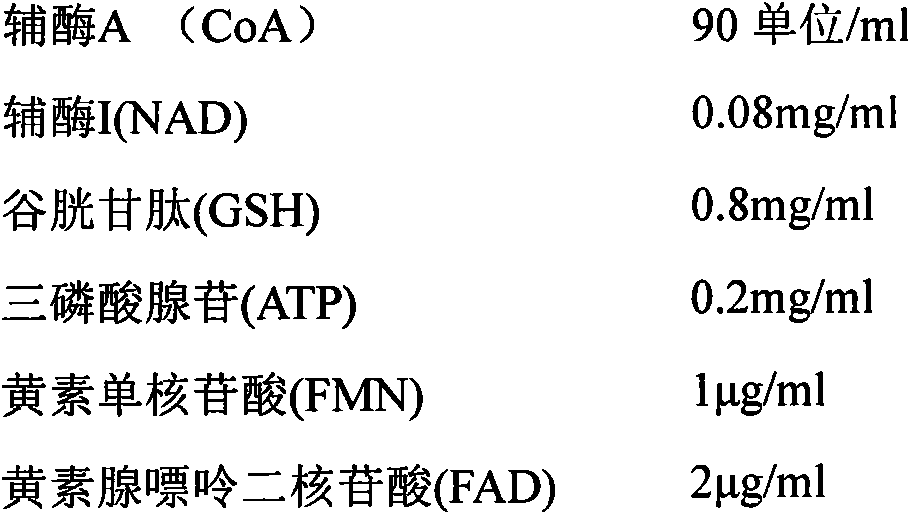
[0044] 1) Take out the frozen fresh yeast, add pure water and stir to mix; heat the suspension to 90-97°C, and immediately cool to 20°C until the cell wall is broken. After fully breaking the wall, centrifuge at 2400G, 4-10°C for 20 minutes to collect the crude supernatant;
[0045] 2) Take the activated carbon corresponding to the supernatant volume of the crude extract and pack it into the column, and wash the equilibrated column with pure water. Adjust the pH value of the crude extract supernatant to 5-5.5 and put it on the column, and elute with ethanol ammonia eluent;
[0046] 3) Evaporate and concentrate the eluate, and perform ion exchange chromatography: adjust the pH value of the concentrated solution to 7.0, dilute it with 2 to 5 times of pure water, put it on an anion exchange column, and elute the sample with 20mM pH8.0 phosphate buffer;
[0047] 4) The eluted sample is ultrafiltered with an ultrafilter with a molecular weight of 5000 Daltons, and the filtrate is ...
Embodiment 2
[0053] 1) Take out the frozen fresh yeast, add pure water and stir to mix; heat the suspension to 90-97°C, and immediately cool to 20°C until the cell wall is broken. After fully breaking the wall, centrifuge at 4390G, 4-10°C for 20 minutes to collect the crude supernatant;
[0054] 2) Take the activated carbon corresponding to the supernatant volume of the crude extract and pack it into the column, and wash the equilibrated column with pure water. The supernatant of the crude extract was adjusted to a pH value of 5-5.5 and put on the column, and eluted with the eluent of ammonia ethanol;
[0055] 3) Evaporate and concentrate the eluate, and perform ion exchange chromatography: adjust the pH value of the concentrated solution to 7.0, dilute it with 2 to 5 times of pure water, put it on an anion exchange column, and elute the sample with 20mM pH8.0 phosphate buffer;
[0056] 4) The eluted sample is ultrafiltered with an ultrafilter with a molecular weight of 5000 Daltons, and ...
Embodiment 3
[0061] 1) Take out the frozen fresh yeast, add pure water and stir to mix; heat the suspension to 90-97°C, and immediately cool it down to 20°C until the cell wall is broken. After fully breaking the wall, centrifuge at 6320G, 4-10°C for 20 minutes to collect the crude supernatant;
[0062] 2) Take the activated carbon corresponding to the supernatant volume of the crude extract and pack it into the column, and wash the equilibrated column with pure water. The supernatant of the crude extract was adjusted to a pH value of 5-5.5 and put on the column, and eluted with the eluent of ammonia ethanol;
[0063] 3) Evaporate and concentrate the eluate, and perform ion exchange chromatography: adjust the pH value of the concentrated solution to 7.0, dilute it with 2 to 5 times of pure water, put it on an anion exchange column, and elute the sample with 20mM pH8.0 phosphate buffer;
[0064] 4) The eluted sample is ultrafiltered with an ultrafilter with a molecular weight of 5000 Dalto...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com