Preparation method of flurbiprofen axetil
A technology of flurbiprofen axetil and synthesis method, which is applied in the field of preparation of flurbiprofen axetil, can solve problems such as short synthesis route, and achieve the effects of convenient operation, environment-friendly, and rich synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
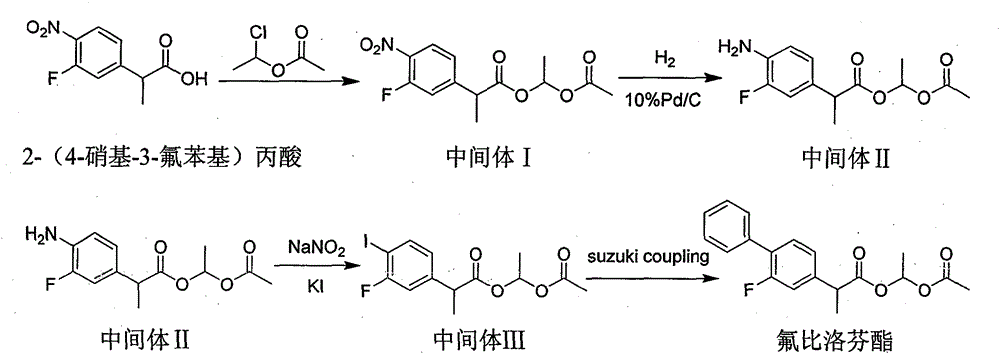
[0028] Example 1 Preparation of 2-(4-nitro-3-fluorophenyl)propionic acid-1-acetoxyethyl ester (intermediate I)
[0029] Add 2-(4-nitro-3-fluorophenyl) propionic acid (213.0 g, 1.000 mol) and anhydrous acetonitrile (2000 ml) into the reactor, replace the air with nitrogen three times, slowly heat up to 35-40 ° C, Further anhydrous potassium carbonate (276.4 g, 2.000 mol) was added. 1-Chloroethyl acetate (245.1 g, 2.000 mol) was added dropwise to the system. After completion, control the internal temperature of the reaction system at 35-40°C for 5.0 hours. The reaction solution was concentrated under reduced pressure until no liquid flowed out. After cooling to 15-25°C, ethyl acetate (2000ml) and water (2000ml) were added, and the layers were separated. The organic phase was concentrated under reduced pressure to obtain 242.4g of a light yellow oil. Yield 81.00%.
Embodiment 2
[0030] Example 2 Preparation of 2-(4-amino-3-fluorophenyl)propionic acid-1-acetoxyethyl ester (intermediate II)
[0031] After diluting 2-(2-fluoro-4-nitro)propionic acid-1-acetoxyethyl ester (149.6g, 0.500mol) with absolute ethanol (900.0ml), add 10% wet palladium carbon (15.00g ), nitrogen replacement for three times, then pass through hydrogen with a pressure of 0.3-0.4 MPa to carry out the reduction reaction, stir at 50-60° C. for 16 h, and then filter off palladium-carbon. The organic phase was concentrated under reduced pressure to obtain 128.0 g of brown oil with a yield of 95.10%.
Embodiment 3
[0032] Example 3 Preparation of 2-(3-fluoro-4-iodophenyl)propionic acid-1-acetoxyethyl ester (intermediate III)
[0033] 2-(4-Amino-3-fluorophenyl)propionic acid-1-acetoxyethyl ester (107.7, 0.400mol), 40% hydrobromic acid (0.440mol) and water (200ml) were added to the reactor, Cool down to 0-5°C, which is system I; control the temperature at 0-5°C, add sodium nitrite (0.440mol) aqueous solution dropwise to system I; make a solution of potassium iodide (0.800mol) and water and cool down to 0-5°C is system II; control the temperature at 0-10°C, add the mixed solution of system I to system II dropwise, slowly raise the temperature to 60-70°C after the dropwise addition, and react for 2-3 hours. After cooling down to 20-30°C, extract twice with dichloromethane, discard the water phase, combine the organic phases, wash the organic phase twice with 10% sodium thiosulfate solution, discard the water phase, and concentrate the organic phase to obtain a brown oil The product was 109....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com