Promethazine hydrochloride tablet and preparation method thereof
A promethazine hydrochloride tablet and a promethazine hydrochloride technology are applied in the directions of anti-inflammatory agents, pill delivery, pharmaceutical formulations, etc., which can solve the problem of uneven content uniformity of active ingredients, save dosage and cost, and achieve compressibility. Good, compliance-increasing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] (1) Preparation of solid dispersion carrier: Add 20 g of Poloxamer 407 and 85 g of PEG6000 into 60% (ml / ml) ethanol of 1 / 3 of the prescription amount, heat and stir until Poloxamer 407 and PEG6000 are completely dissolved That is, the auxiliary material solution is obtained;
[0018] Promethazine hydrochloride (dry, pure) 250g is dissolved in 60% (ml / ml) ethanol of recipe quantity 1 / 3 to obtain main drug solution; Gained adjuvant solution and main drug solution are mixed uniformly to obtain mixed solution; Gained mixing The solution is dried in vacuum until the water content is less than 1% (mass percentage);
[0019] (2) Preparation of tablet: pulverize the material obtained in step (1) to 100 mesh to obtain a solid dispersion powder; put the gained solid dispersion powder, starch 180g, and microcrystalline cellulose 360g into a granulator, and dry mix Evenly, add 60% (ml / ml) ethanol of the remaining 1 / 3 of the prescription amount as a binder, set the shearing knife f...
Embodiment 2
[0021] (1) Preparation of solid dispersion carrier, add poloxamer 407 25g and PEG6000 80g into 60% (ml / ml) ethanol of 1 / 3 of the prescription amount, heat and stir until poloxamer and PVPk30 are completely dissolved Yes, excipient solution;
[0022] Promethazine hydrochloride (dry, pure) 250g is dissolved in 60% (ml / ml) ethanol of recipe quantity 1 / 3 to obtain main drug solution; Gained adjuvant solution and main drug solution are mixed uniformly to obtain mixed solution; Gained mixing The solution is dried in vacuum until the water content is less than 1% (mass percentage);
[0023] (2) Preparation of tablet: pulverize the material obtained in step (1) to 120 mesh to obtain solid dispersion powder; put the gained solid dispersion powder, starch 180g, and microcrystalline cellulose 360g into a granulator, and dry mix Evenly, add 60% (ml / ml) ethanol of the remaining 1 / 3 of the prescription amount as a binder, set the frequency of the shearing knife as 30 Hz, and shear and gran...
Embodiment 1、2
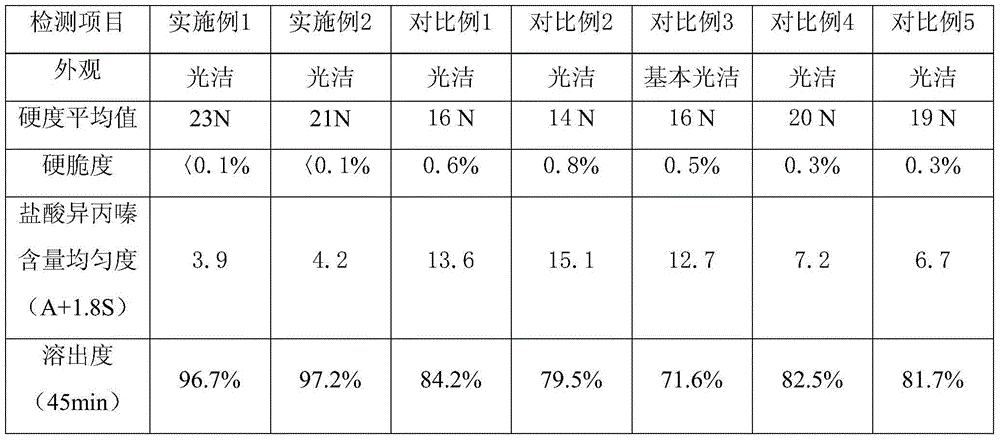
[0034] Embodiment 1, 2, and the outward appearance of product in each comparative example, content uniformity are as shown in table 1 (check by Chinese Pharmacopoeia 2010 editions)
[0035] Table 1
[0036]
[0037] (Dissolution is tested according to the standards in Appendix XC of Part Two of the Chinese Pharmacopoeia 2010 Edition).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com