Polymer material containing multiple conjugated chain sections as well as preparation method and application of polymer material
A technology of polymers and conjugated chains, applied in the field of organic polymer optoelectronic materials, can solve the problems of easy crystallization, poor film formation, lack of optoelectronic materials, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0103] Preparation of Segmented Conjugated Polymers
[0104] The segmented conjugated polymer can be prepared by conventional methods in the art, such as by the following method:
[0105] (1) preparing a polymerized monomer, characterized in that the polymerized monomer has a conjugated segment and a non-conjugated segment located at one or both ends of the conjugated segment and connected to the conjugated segment, and the The end of the non-conjugated segment has a functional group (such as hydroxyl, amine, aldehyde, carboxyl, isocyanate, azide, halo, alkenyl or alkynyl, etc.) for polymerization to form a covalent bond ); wherein, the conjugated segment is a fully conjugated structure;
[0106] (2) Polymerization is carried out using the polymerizable monomer, thereby forming the polymer of the present invention.
[0107] Wherein, the reaction may be (but not limited to): urethanization reaction, esterification reaction, amidation reaction, imidization condensation reactio...
Embodiment 1
[0113] Polyurethane {[2,5-bis(2-octyldodecyl)-3,6-bis[11-(4-terthiophene-phenolyl)-1-undecyl]ol-2-yl-pyrrole And[3,4-c]pyrrole-1,4-dione]-hexamethylene diisocyanate}(PU_DPP C 20 -Synthesis of HMDI)
[0114]
[0115] (1) 2,5-bis(2-octyldodecyl)-3,6-bis{5″-[4-(11-hydroxy-undecyloxy)phenyl]-((2″ Synthesis of ,5′)-(2′,2)-terthiophene)-5-yl}-2-yl-pyrrolo[3,4-c]pyrrole-1,4-dione
[0116] (a) 2,5-bis(2-octyldodecyl)-3,6-bis(5-bromo-thiophene)-2-yl-pyrrolo[3,4-c]pyrrole-1,4 - Synthesis of diketones
[0117] 3,6-Dithiophen-2-yl-pyrrolo[3,4-c]pyrrole-1,4-dione (25mmol, 7.5g) and potassium carbonate (163mmol, 25g) were added into a 500mL Schleck reaction flask. After repeated evacuation and filling with argon, the atmosphere in the reaction bottle was changed to an argon atmosphere, and 150 mL of N,N-dimethylformamide was injected. Heat to 145°C with stirring, add 2-octyl dodecyl bromide (100mmol, 36.15g) after the temperature stabilizes, and react the system at 145°C for 20 hour...
Embodiment 2
[0129] Polyurethane {[2,5-bis(2-octyldodecyl)-3,6-bis[11-(4-terthiophene-phenolyl)-1-undecyl]ol-2-yl-pyrrole And[3,4-c]pyrrole-1,4-dione]-m-phenylene diisocyanate}(PU_DPPC 20 -synthesis of mPhDI)
[0130]
[0131] The synthesis method is the same as that of PUDPP C 20 - HMDI polymers, except that m-phenylene diisocyanate is used instead of hexamethylene diisocyanate in the raw material.
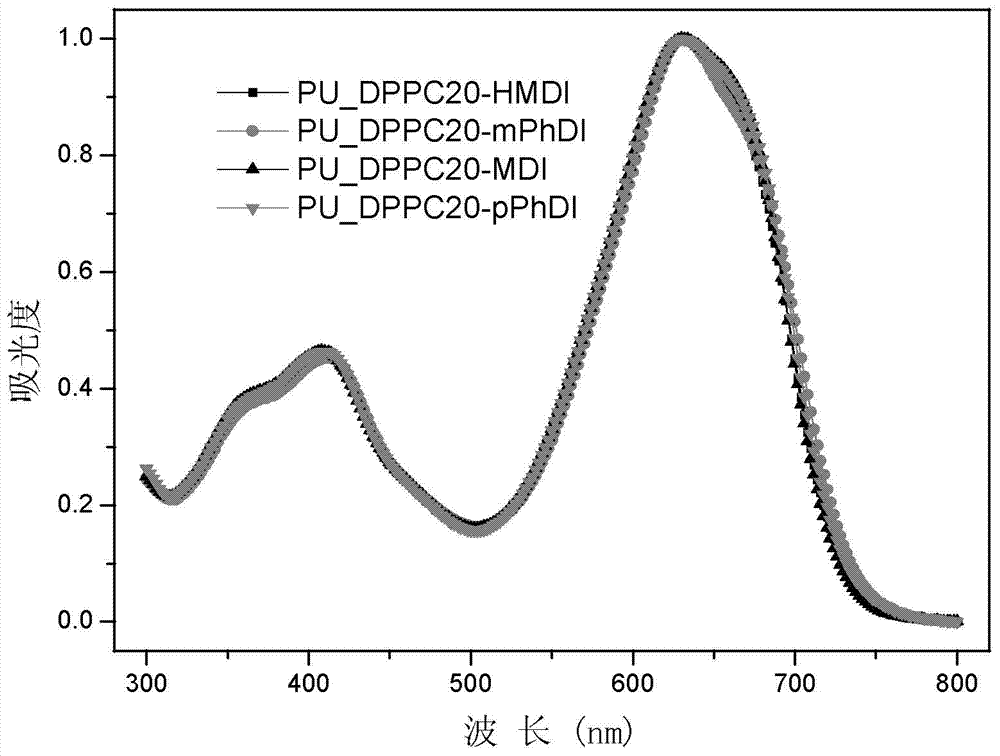
[0132] GPC: M n =6656,M w =9369,M w / M n =1.41;UV(CHCl 3 ):λ max =411,632nm; HOMO=-5.31eV, LUMO=-3.72eV.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com