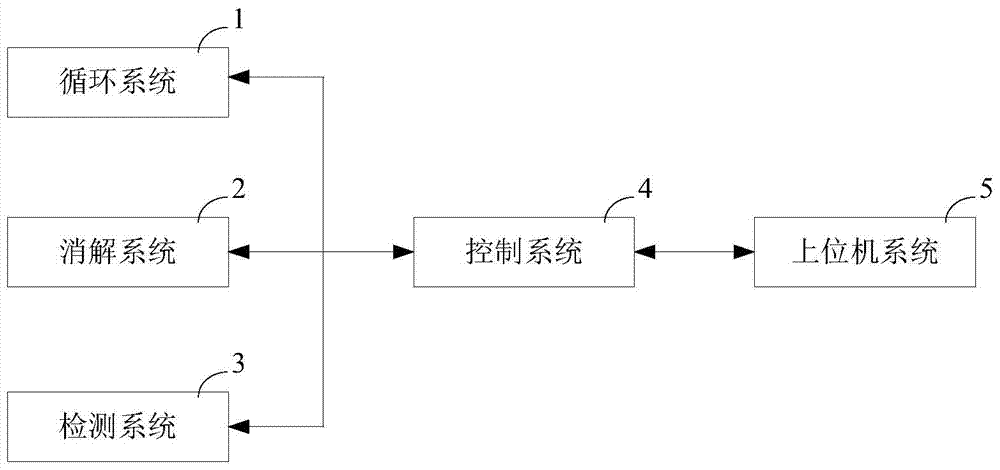
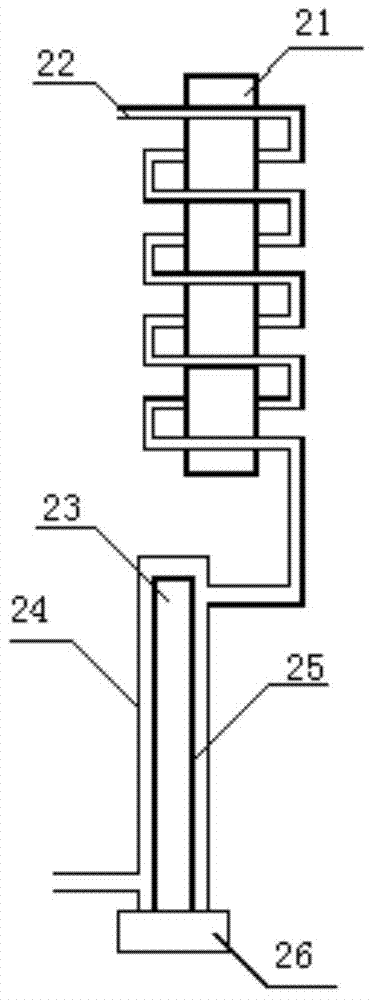
Water quality monitoring instrument and method
A water quality monitoring and instrument technology, applied in the field of total phosphorus and ammonia nitrogen water quality online monitoring instruments, can solve the problems of high cost, inability to simultaneously detect water quality total phosphorus concentration and ammonia nitrogen concentration online, and low efficiency, etc., to improve popularity, low cost and real-time The effect of prior monitoring
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
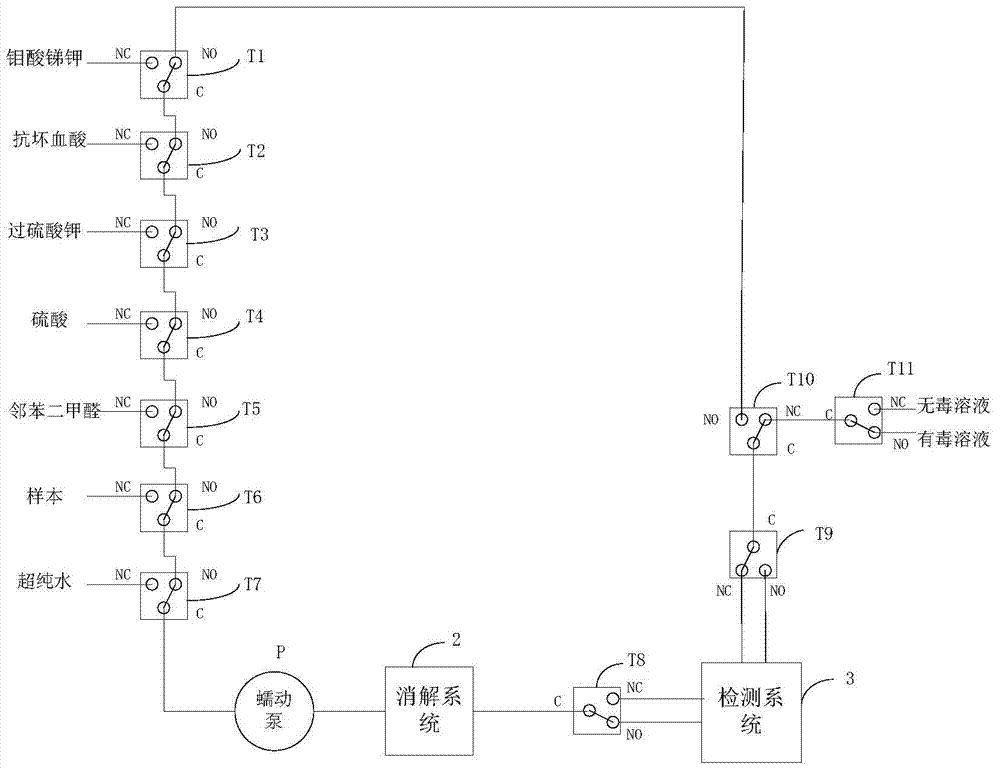
[0077] Step 1, when detecting total phosphorus, heat the water sample to 110°C, irradiate it with ultraviolet light with a wavelength of 254nm for 40 minutes, wherein, the water sample contains organic phosphorus or / and polymeric phosphate, and add over Potassium sulfate solution, catalytic reduction of water samples into orthophosphate solution;
[0078] In step 2, the orthophosphate solution digested by ultraviolet light is sequentially added to an antimony potassium molybdate solution and an ascorbic acid solution to prepare a phosphomolybdenum blue solution;
[0079] Step 3, under the irradiation of the first light source 31, the absorbance value of the phosphomolybdenum blue solution in the color reaction is obtained, and the content of total phosphorus in the water sample is calculated according to the absorbance value and the pre-stored absorption spectrum colorimetric.
Embodiment 2
[0081] Step 1, when detecting total phosphorus, heat the water sample to 120°C, irradiate it with ultraviolet light with a wavelength of 254nm for 30 minutes, wherein the water sample contains organic phosphorus or / and polymeric phosphate, and add over Potassium sulfate solution, catalytic reduction of water samples into orthophosphate solution;
[0082] In step 2, the orthophosphate solution digested by ultraviolet light is sequentially added to an antimony potassium molybdate solution and an ascorbic acid solution to prepare a phosphomolybdenum blue solution;
[0083] Step 3, under the irradiation of the first light source 31, the absorbance value of the phosphomolybdenum blue solution in the color reaction is obtained, and the content of total phosphorus in the water sample is calculated according to the absorbance value and the pre-stored absorption spectrum colorimetric.
Embodiment 3
[0085] Step 1, when detecting total phosphorus, heat the water sample to 130°C, irradiate it with ultraviolet light with a wavelength of 254nm for 20 minutes, wherein the water sample contains organic phosphorus or / and polymeric phosphate, and add over Potassium sulfate solution, catalytic reduction of water samples into orthophosphate solution;
[0086] In step 2, the orthophosphate solution digested by ultraviolet light is sequentially added to an antimony potassium molybdate solution and an ascorbic acid solution to prepare a phosphomolybdenum blue solution;
[0087] Step 3, under the irradiation of the first light source 31, the absorbance value of the phosphomolybdenum blue solution in the color reaction is obtained, and the content of total phosphorus in the water sample is calculated according to the absorbance value and the pre-stored absorption spectrum colorimetric.
[0088] Among them, such as Figure 7 As shown, the ammonia nitrogen detection flow chart in the wat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The inside diameter of | aaaaa | aaaaa |
| Wall thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com