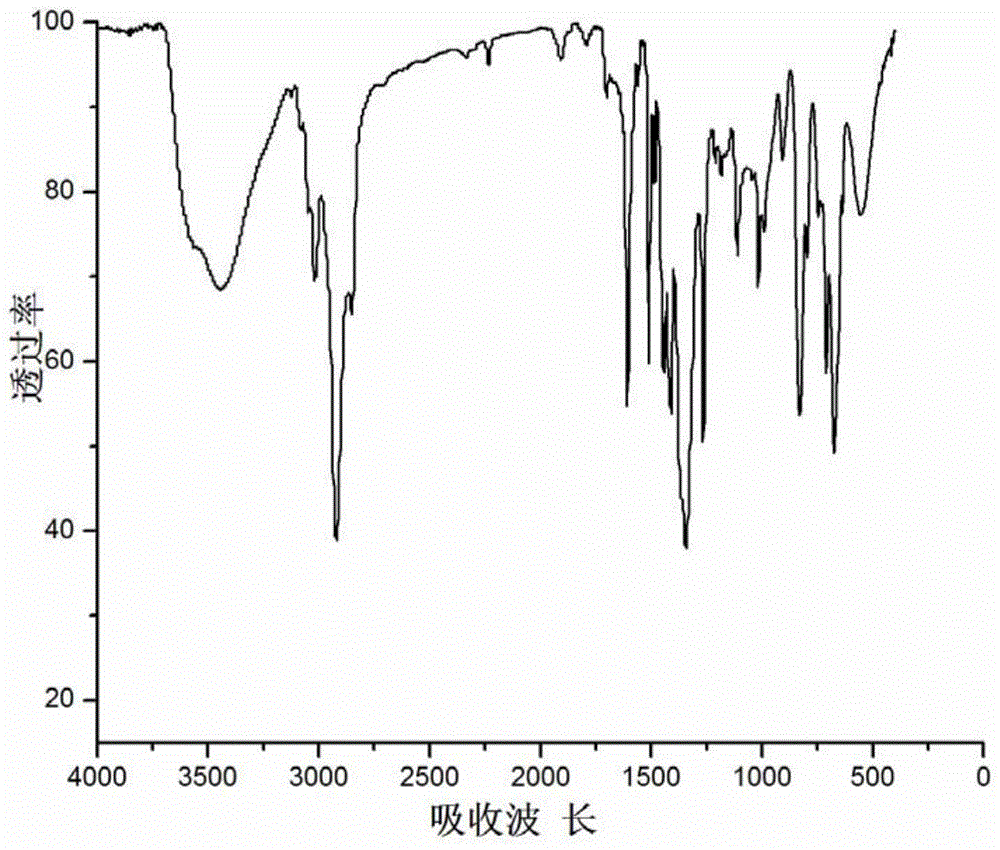
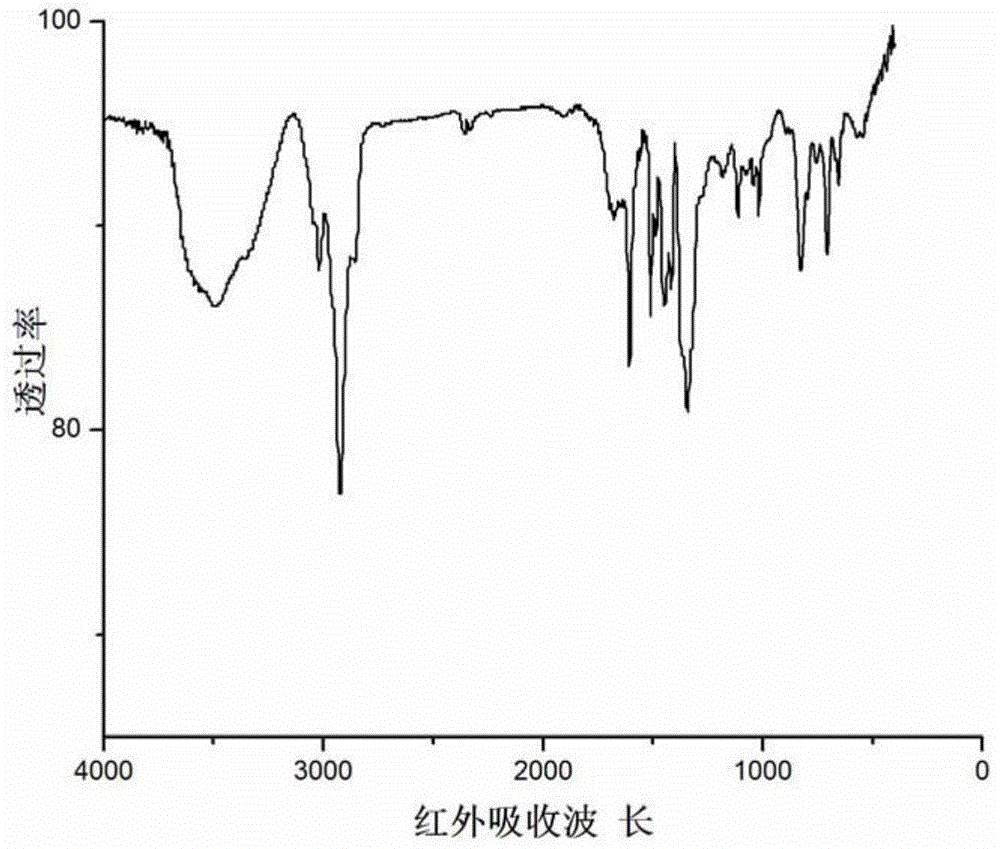
Phenylboronic acid-modified covalent affinity hypercrosslinked resin, and preparation method and application thereof
A technology of ultra-high cross-linked resin and phenylboronic acid, which is applied in the field of resin to achieve the effects of good adsorption performance, simple preparation steps, and avoidance of the activation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The preparation of the covalent affinity ultra-high cross-linked resin modified by phenylboronic acid comprises the following steps:
[0035] (a) In a 500ml three-neck flask equipped with a reflux condenser, a thermometer and a nitrogen protection device, add 400ml of acetonitrile, and cool the system to 0°C in an ice bath;
[0036] (b) Add 0.25g of azobisisobutyronitrile, 2.98ml of divinylbenzene, 3.81ml of 4-chloromethylstyrene and 1.2g of 4-vinylbenzeneboronic acid to the ice-water bath system, in which 4-ethylene Phenylboronic acid needs to be dissolved in 40ml of absolute ethanol and then added to the reaction system. The temperature of the system is maintained at 0°C, and the nitrogen gas is blown for 2 hours to remove the oxygen in the system;
[0037] (c) Then transfer the reaction to an oil bath, adjust the magnetic stirrer to an appropriate rotation speed, and slowly raise the system from room temperature to 70°C (according to a heating rate of 4min / °C), and m...
Embodiment 2
[0047] The covalent affinity ultra-high cross-linked resin of this example is used as a solid phase extraction filler to separate catechol (cis-diol type) and hydroquinone (non-cis-diol type) isomers, the steps for:
[0048] (a) Preparation of solvent: Accurately weigh 0.1750 g of disodium hydrogen phosphate, and set the volume to 500 ml to obtain pH=8.5 phosphate buffer A. Accurately pipette 0.5ml of trifluoroacetic acid and 15ml of acetonitrile, and dilute to 50ml with ultrapure water to obtain solution B containing 1% trifluoroacetic acid in acetonitrile:water=30:70. Accurately pipette 10 ml of acetonitrile, and dilute to 100 ml with ultrapure water to obtain acetonitrile solution C of acetonitrile:water=10:90.
[0049] (b) Prepare the solution to be tested: Accurately weigh 0.2000 g of catechol and hydroquinone, and use the solutions prepared in step (a) to make up to 50 ml respectively.
[0050] (c) Packing of solid-phase extraction column: Accurately weigh 60 mg of cov...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Height | aaaaa | aaaaa |
| The inside diameter of | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com