Method for treating stainless steel pickling waste liquor and recovering iron, chromium and nickel
A technology of pickling waste liquid and recovery method, which is applied in metallurgical wastewater treatment, chemical instruments and methods, water/sewage multi-stage treatment, etc., can solve the problems of low recovery rate and high treatment cost, and achieve low equipment cost and excellent treatment process Simple and easy to operate, the effect of objective economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
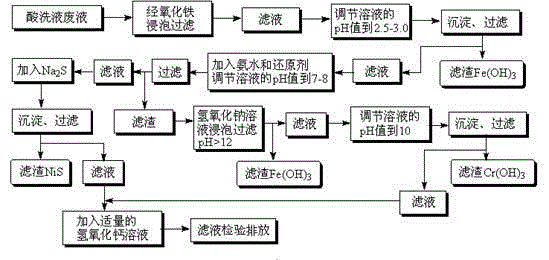
[0024] Get stainless steel pickling waste liquid 100mL and pour in 500mL beaker, the concentration of ferric ion is about 75mg / L after measuring, and chromium (III) ion concentration is 20mg / L, and nickel ion concentration is 18mg / L.
[0025] First use iron oxide scale to neutralize part of the acid in the waste liquid, after filtering, use 1 mol / L sodium hydroxide solution to adjust the pH value to 2.8, and obtain the preliminary separated Fe(OH) after filtering again 3 precipitation. In the filtrate, add ammonia solution and 1g of sodium sulfite with a molar ratio of 6:1 to nickel ions, adjust the pH value to 7.8, and obtain a solution containing Cr(OH) after filtration. 3 and a small amount of Fe(OH) 3 Filter residue 1 and filtrate 1 of nickel element. The filter residue 1 is adjusted to pH 12.2 through 1 mol / L sodium hydroxide solution, and the secondary separated Fe(OH) is obtained after precipitation and filtration. 3 Precipitate and chromium (III) ion filtrate, use 1...
Embodiment 2
[0027] Get stainless steel pickling waste liquid 100mL and pour in 500mL beaker, the concentration of ferric ion is about 79mg / L after measuring, chromium (III) ion concentration is 23mg / L, and nickel ion concentration is 19mg / L. The difference from Example 1 is that sodium bisulfite is used as a reducing agent, and the adjustment of pH value is different from Example 1 according to the dosage of ammonia and sodium hydroxide solution added.
[0028] First use iron oxide scale to neutralize part of the acid in the waste liquid, after filtering, use 1 mol / L sodium hydroxide solution to adjust the pH value to 2.5, and obtain the preliminary separated Fe(OH) after filtering again 3 precipitation. In the filtrate, add ammonia solution and 1.5g sodium bisulfite in a molar ratio of 6:1 to nickel ions, adjust the pH value to 8, and obtain a solution containing Cr(OH) after filtration. 3 and a small amount of Fe(OH) 3 Filter residue 1 and filtrate 1 of nickel element. The filter res...
Embodiment 3
[0030] Different from Example 1 is to adopt the stainless steel pickling liquid waste water that laboratory simulation is configured into, and wherein use raw material is Cr(NO 3 ) 3 , Ni(NO 3 ) 2 and Fe(NO 3 ) 3 , take an appropriate amount of weight and add deionized water and hydrochloric acid to adjust the pH value to 2.2, and the concentration of chromium and nickel ions in the solution is 50mg / L, and the concentration of iron ions is 100mg / L. Take 100mL of the prepared solution and pour it into a 500mL beaker.
[0031] Directly use 1 mol / L sodium hydroxide solution to adjust the pH value to 2.5, and obtain the preliminary separated Fe(OH) after filtration 3precipitation. In the filtrate, add ammonia solution and 3g of sodium sulfite in a molar ratio of 6:1 to nickel ions, adjust the pH to 7.8, and obtain a solution containing Cr(OH) after filtration. 3 and a small amount of Fe(OH) 3 Filter residue 1 and filtrate 1 of nickel element. The filter residue 1 is adjus...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com