Open-loop rubiaceae-type cyclopeptide, pharmaceutical composition taking open-loop rubiaceae-type cyclopeptide as active ingredient as well as preparation method and application of open-loop rubiaceae-type cyclopeptide
A technology of Rubiaceae and composition, which is applied in the field of ring-opening Rubiaceae type cyclic peptides, can solve problems such as insufficient extraction, and achieve the effects of good controllability and reproducibility, convenient operation, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
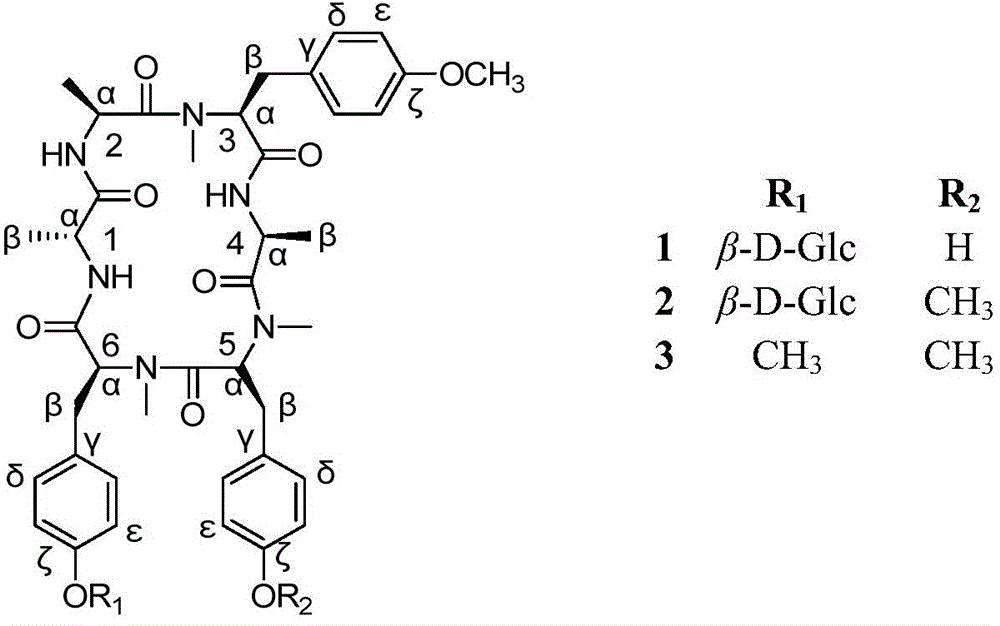
[0029] Preparation and structural identification of the ring-opening Rubiaceae-type cyclic peptide compounds rubicordilin A(1), rubicordilin B(2) and rubicordilin C(3):
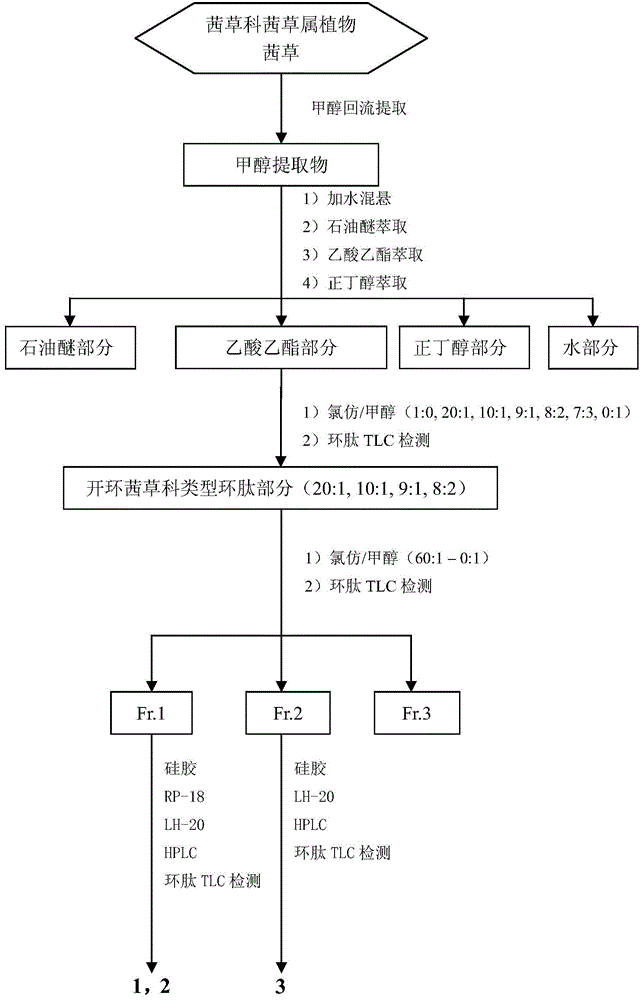
[0030] Take madder root and rhizome (200kg), after drying and crushing, reflux extraction with industrial methanol for 3 times (300L×3 times), 3 hours each time. The extract was concentrated under reduced pressure to obtain 29.2 kg of methanol extract. After the methanol extract was heated and suspended in water, the insoluble matter was filtered off, extracted 3 times with petroleum ether, ethyl acetate and n-butanol successively, and each part of the extract was concentrated under reduced pressure to obtain the petroleum ether part (2.6kg), ethyl acetate Ester fraction (4.0 kg), n-butanol fraction (3.0 kg) and water fraction (14.0 kg). The ethyl acetate fraction (4.0kg) was subjected to silica gel column chromatography and eluted with chloroform / methanol gradient (100:0, 20:1, 10:1, 9:1, 8:2, 7:3, 0:100 )...
Embodiment 2
[0054] The open-loop Rubiaceae type cyclic peptide compound rubicordilin A (1), rubicordilin B (2) and rubicordilin C (3) of the present invention are determined by SRB method in its human non-small cell lung cancer cell line (A549), human gastric cancer cell line (SGC- 7901) and human cervical cancer cells (HeLa), and found that the split-ring Rubiaceae type cyclic peptide compound has better cytotoxic activity. The experimental principles, methods and results are as follows:
[0055] Experimental principle: After co-cultivating the drug with the cells, the cell survival rate is measured. SRB is a water-soluble protein dye with a sulfonic acid group anion in the molecule, which can combine with basic amino acids of intracellular proteins under weakly acidic conditions. Dissolve SRB in cells with alkaline solution and measure its absorbance value. SRB The content of reflects the protein content in the cell, which reflects the cell viability.
[0056] Experimental method: Cel...
Embodiment 3
[0062] The open-loop Rubiaceae type cyclic peptide compounds rubicordilin A (1), rubicordilin B (2) and rubicordilin C (3) of the present invention are tested for their NF-κB signaling pathway inhibitory activity by the luciferase double reporter gene method, and it is found that the cyclic peptide compounds can Inhibits NF-κB pathway activity. The experimental principles, methods and results are as follows:
[0063] Experimental principle: The luciferase dual reporter gene system is often used to evaluate the effect of compounds on the activity of a certain signaling pathway. Luciferin emits fluorescence under the catalysis of luciferase, and the luminescence intensity is detected by a chemiluminescence microplate reader to reflect the activity of the pathway. strong and weak. NF-κB-dependent firefly luciferase plasmid (5×κB-luciferase) was transiently transfected in HEK 293T cells to specifically reflect the activity of NF-κB pathway, and Renilla luciferase plasmid (pTK-Ren...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com