Polypeptide binding with tumor necrosis factor, and applications thereof
A tumor necrosis factor and polypeptide inhibitor technology, applied in the field of biomedicine, can solve the problems of good curative effect, aggravation of cardiac insufficiency, high drug cost, etc., and achieve the effect of inhibiting cell apoptosis, reducing effective concentration, and reducing treatment cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
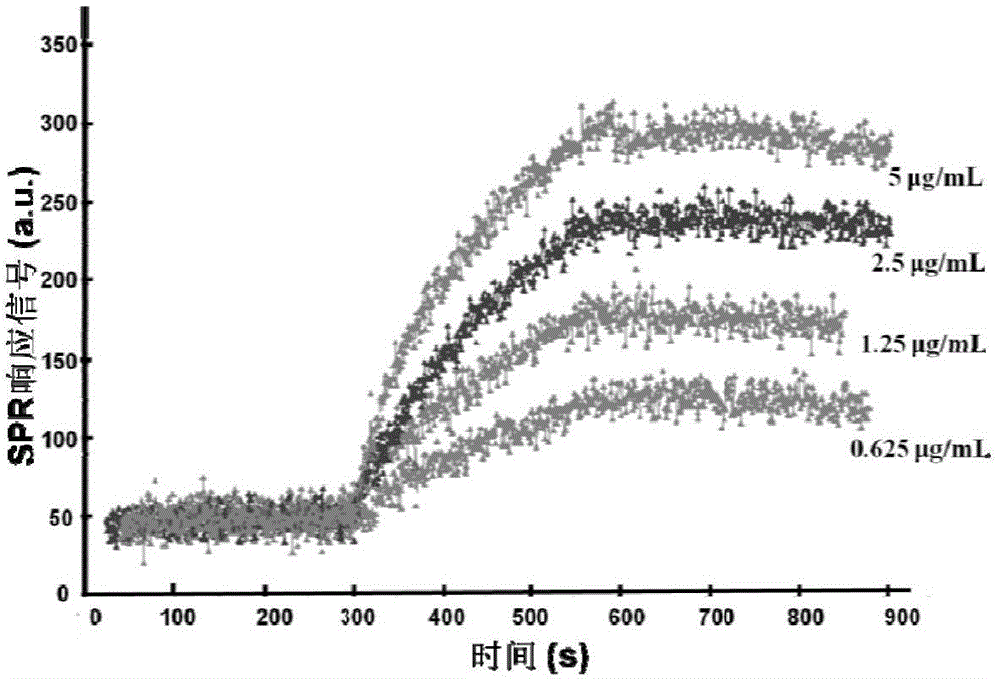
[0075] The determination of the affinity of embodiment 1 polypeptide to TNF-α
[0076] (1) Prepare the polypeptide with 1×PBS to a solution with a concentration of 1 mg / mL, and prepare the anti-TNF-α polypeptide to a solution with a concentration of 100 μg / mL, take 2.5 μL and drop it on a carboxylated SPR chip (purchased from Plexera company, Kx5 type SPR standard substrate), and the polypeptide was immobilized on the SPR chip through the specific reaction of streptavidin and biotin. Then the TNF-α solution (diluted in 1×PBS) with a concentration of 0.625 μg / mL, 1.25 μg / mL, 2.5 μg / mL, and 5 μg / mL was passed through the chip surface as the mobile phase, the binding time was 150 s, and the dissociation time was 150 s. The respawn time is 250s. 1×PBS was used as the dissociation solution, and 0.5% phosphoric acid was used as the reconstitution solution. Record the binding-dissociation curve of the polypeptide and TNF-α on the K×5 SPR instrument (Plexera), such as figure 1 show...
Embodiment 2
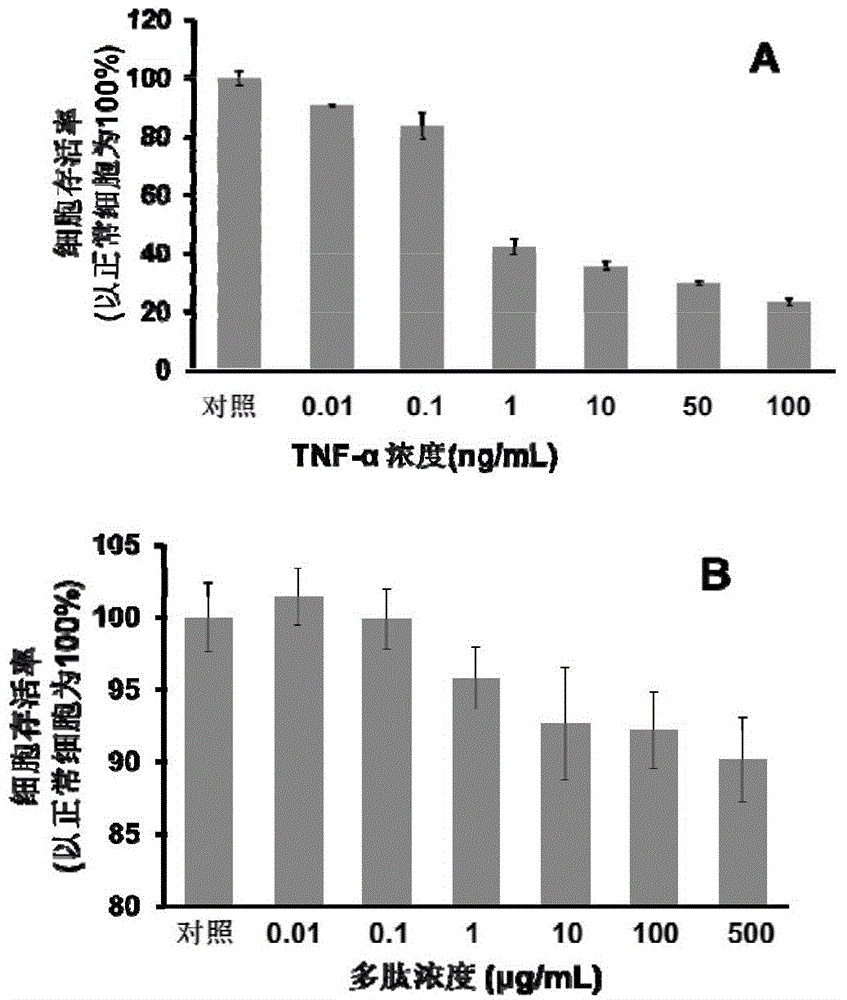
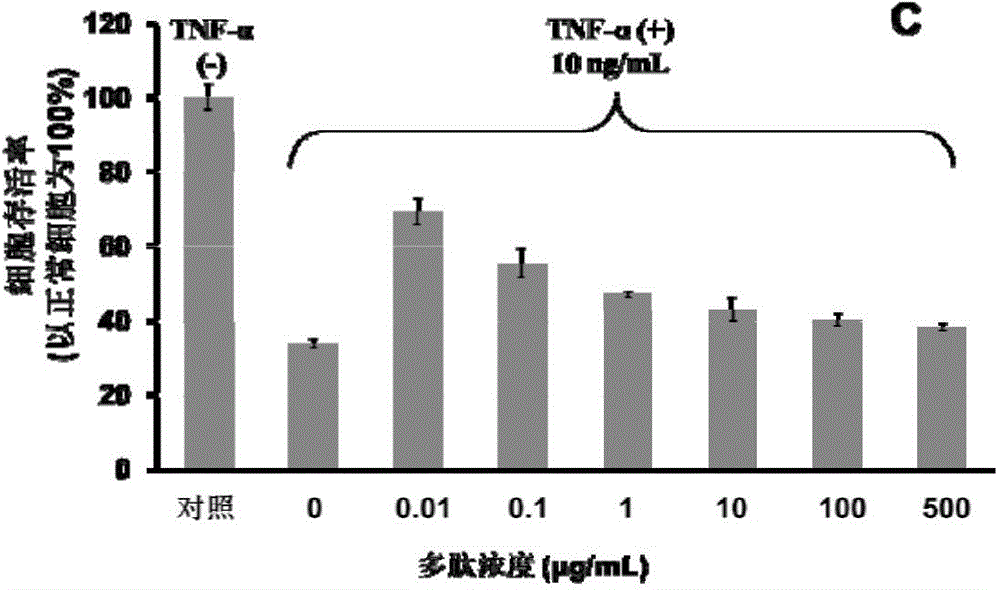
[0080] Example 2 The MTT method evaluates the effect of the polypeptide on the cytotoxicity induced by TNF-α
[0081] The mouse fibroblast cell line L929 was used as a research model, and the cells were cultured in RPMI-1640 medium (containing 10% North American fetal bovine serum, 1% penicillin and streptomycin). In a 96-well cell culture plate (Corning), each well was cultured with 1×10 4 12 cells, after 12 hours in the cell plate, add a series of concentration gradient TNF-α and the polypeptide in the culture plate (the specific concentration can be seen figure 2 ). The final volume of culture medium in each well was 200 μL. After incubation for 48 hours, 20 μL of thiazolium blue (MTT) 1×PBS solution with a concentration of 5 mg / mL was added to each well of the culture plate. React at 37°C for 4 hours. The solution in the culture plate was sucked away, and 100 μL of dimethyl sulfoxide (DMSO) was added to each well to dissolve the formazan precipitate. In a continuous sp...
Embodiment 3
[0083] Example 3 Observation of the Effect of the Polypeptide on TNF-α-Induced Cell Quantity Reduction Using an Optical Microscope
[0084] The cell culture method was as described in Example 2, except that a 6-well plate was used for cell culture, and the final volume of culture medium in each well was 2 mL.
[0085] After TNF-α and the polypeptide were added to the cells and incubated for 48 hours, observed and photographed under an optical microscope, as shown in image 3 shown. (A) is the untreated cells, (B) is the cells treated with 10ng / mL TNF-α. Compared with the normal cultured cells, it can be found that in the same area, the number of cells is greatly reduced, and apoptosis can be seen. Apoptotic bodies; (C-E) were co-incubated with TNF-α at a concentration of 0.1ng / mL, 10ng / mL, and 1000ng / mL, and it was found that 0.1ng / mL of polypeptide had no significant effect on cell proliferation , when the concentration of the polypeptide increased to 10ng / mL, the number of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Equilibrium dissociation constant | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com