Acinetobacter baumannii zinc dependent oligopeptide A1S-1610 recombinant protein and preparation method and application thereof
A technology of Acinetobacter baumannii and protein, applied in the biological field, can solve the problems of toxic and side effects, large toxic and side effects, complex vaccine components of the outer membrane complex, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
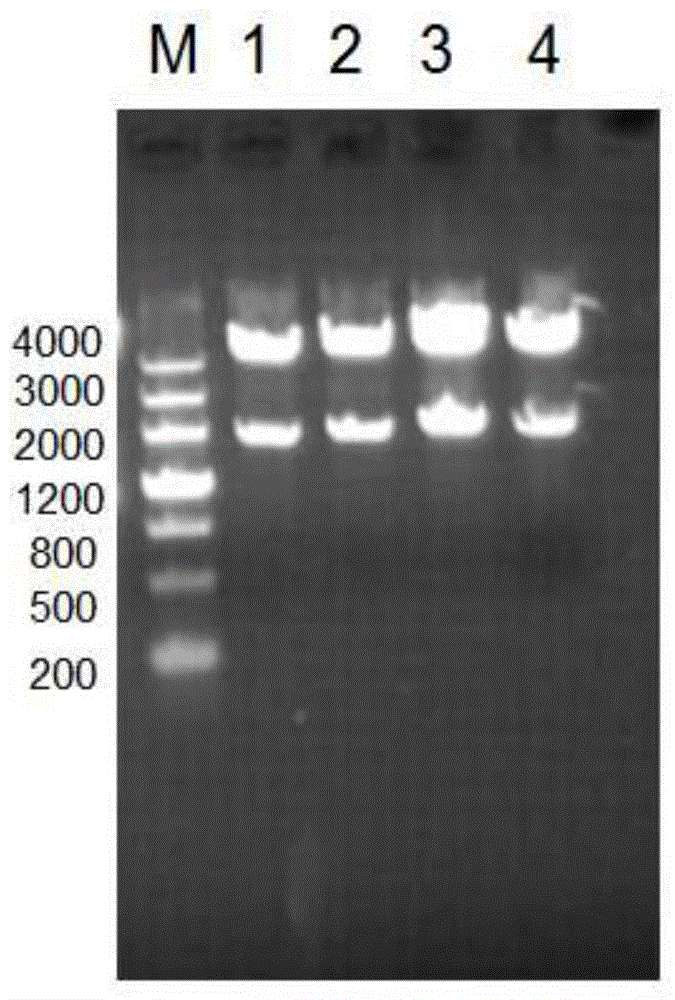
[0058] Embodiment 1: Cloning of Acinetobacter baumannii A1S_1610 protein
[0059] 1. Firstly, according to the gene sequence of Acinetobacter baumannii 17978 standard strain A1S_1610, the signal peptide analysis software was used to analyze the results. Image 6 .
[0060] 2. According to the analysis results, use the PCR method to amplify the gene fragment of A1S_1610 protein with the whole genome of Acinetobacter baumannii 17978 as a template. The amplification steps are as follows:
[0061] 1) Design the PCR primers as follows, which are SEQ ID NO.7-8 (the base sequence of the restriction site is underlined)
[0062] Forward primer PA1S1610B1 (SEQ ID NO.7):
[0063] 5'-CGC GGATCC GAAGCGACGCGTGCGACA-3'
[0064] BamH Ⅰ
[0065] Reverse primer PA1S1610N2 (SEQ ID NO.8):
[0066] 5'-TTAT GCGGCCGC CTTATTTAACCCGTTGTCCGGTAA-3'
[0067] Not Ⅰ
[0068] In this example, the DNA sequence SEQ ID NO.2 encoding the amino acid sequence of the A1S_1610 protein shown in SEQ ID NO.1 ...
Embodiment 2
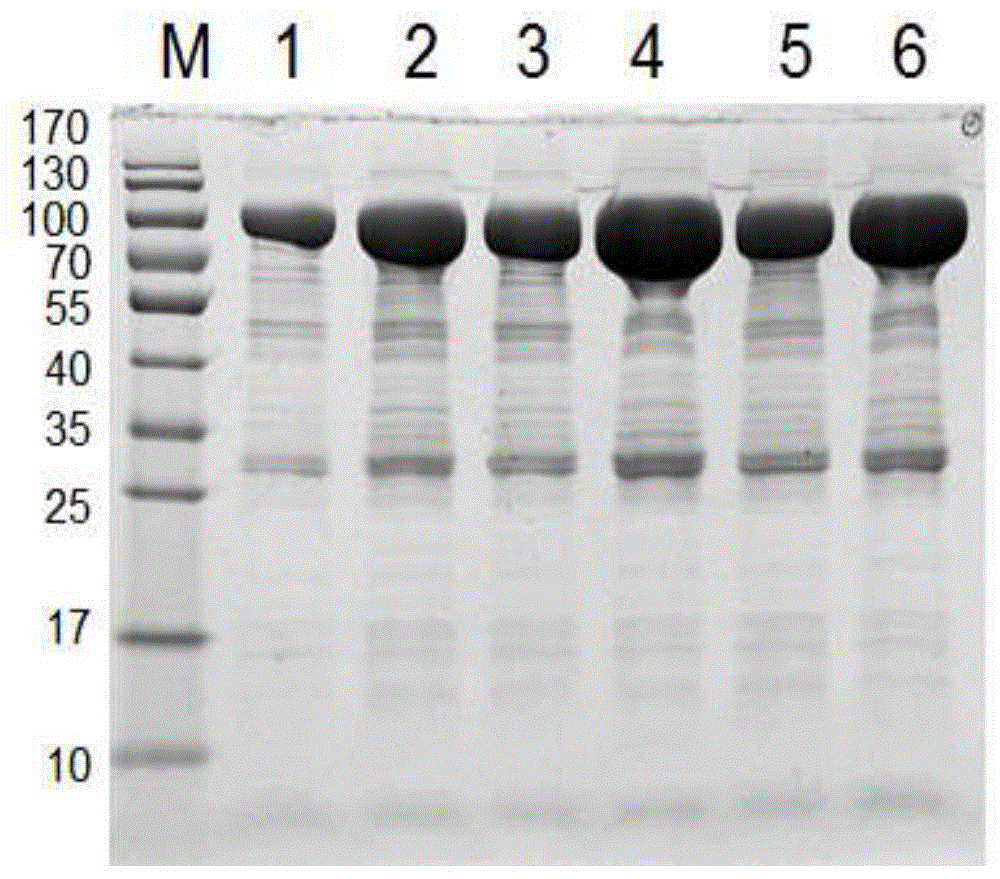
[0098] Example 2: Acinetobacter baumannii-17978A1S_1610 protein induced expression, purification and identification of expression form in prokaryotic expression system-Escherichia coli
[0099] 1. Induced expression of target protein
[0100] 1) Take 100 μL of the pGEX-6P-2-A1S_1610 / XL-1blue bacterial solution that was correctly identified by double enzyme digestion and add it to 10 mL of Amp-resistant TB medium, cultivate overnight at 100 rpm at 37°C, take 2 ml of the overnight cultured bacterial solution and add it to 18 mL In the Amp-resistant TB medium (the rest of the bacterial solution is stored in a 4°C refrigerator for later use), culture at 37°C for 2 to 3 hours at a rotation speed of 250rpm, and after secondary activation until the OD600 is 0.8 to 1.2, add 4.4 μL of IPTG to make it The final concentration was 200 μM, and then placed on a shaker to induce expression at 30°C for 3h, at 25°C for 5h, and at 16°C overnight to induce expression.
[0101] 2) Take out the b...
Embodiment 3
[0106] Embodiment 3: Preparation of A1S_1610 protein antigen
[0107] 1. Amplify culture to obtain protein
[0108] Inoculate the pGEX-6P-2-A1S_1610 / XL-1blue bacteria stored in the -80°C refrigerator on the LB ampicillin-resistant plate, and cultivate overnight at 37°C; pick a single colony and inoculate it in 100ml LB ampicillin-resistant medium, 37 Cultivate overnight at 200 rpm; add 100ml of the activated bacterial solution to 2L LB medium containing Amp resistance for secondary activation, culture at 37°C for 3-4 hours until the OD600 is 1.2, add 420ml of IPTG (final concentration is 200uM) After induction in a shaker at 30°C for 3 hours, centrifuge at 6000rpm for 5 minutes to collect the bacteria, add 80ml of PBS to resuspend the bacteria, then ultrasonically lyse the bacteria for 30 minutes, collect the supernatant and 4ml of glutathione-agarose by centrifugation as above Gel 4B binding; a large amount of A1S_1610 fusion protein containing GST tag was obtained.
[0109...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com