A kind of method of synthesizing n-propyl benzene
A technology of n-propylbenzene and benzyl chloride, applied in the field of synthesizing n-propylbenzene, can solve problems such as slow reaction speed and low efficiency, and achieve the effects of low production cost, simple reaction and low reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
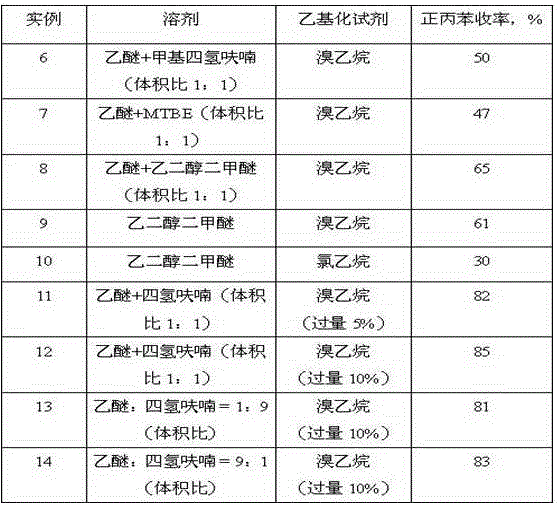
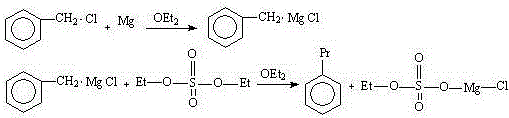
[0031] Embodiment 1: diethyl ether is made solvent, and benzyl chloride reacts with magnesium powder, then reacts with diethyl sulfate to synthesize n-propylbenzene, and concrete steps are as follows:
[0032] In a 500ml four-necked glass flask with a condensing reflux device, a thermometer, a heating device, a stirring device, and a constant pressure dropping funnel, add 5.2 grams of magnesium powder (0.22mol, an excess of 10%) and 30ml of ether; then add benzyl chloride Mix 26.58 g (0.2 mol) and 70 ml of diethyl ether to form a benzyl chloride solution, add it to a constant pressure dropping funnel, and drop 5 ml of benzyl chloride solution into the flask; add 1 iodine crystal to the flask. Heating initiates the Grignard reaction, and starts stirring to fully mix the materials in the flask. Add the remaining benzyl chloride solution dropwise to the flask, and adjust the rate of addition so that the solvent in the material is in a state of slow reflux.
[0033] After 20 minu...
Embodiment 2
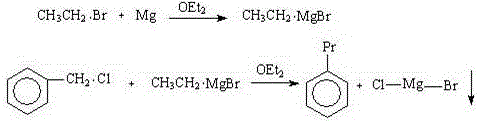
[0035] Example 2: On the basis of Example 1, the solvent was changed to tetrahydrofuran, and the reaction result was (deducting the solvent peak of ether): 8% toluene, 70% n-propylbenzene, and 11% diphenylethane. The boiling point of tetrahydrofuran is high, so that the reaction temperature increases, the selectivity of n-propylbenzene becomes better, and the coupling of benzyl chloride itself is accelerated, and the content of by-product diphenylethane also increases.
Embodiment 3
[0036] Example 3: On the basis of Example 1, the solvent was changed to a mixed solvent of diethyl ether and tetrahydrofuran (each accounting for 50%), and the reaction result was (deducting the diethyl ether solvent peak): 9% toluene, 74% n-propylbenzene, diphenylethyl Alkanes 8%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| flash point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com