Preparation method of proton pump inhibitor enteric-coated tablet
A proton pump inhibitor and proton pump technology, which is applied in the field of pharmacy, can solve problems such as low solubility, and achieve the effect of stable product quality and good market prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
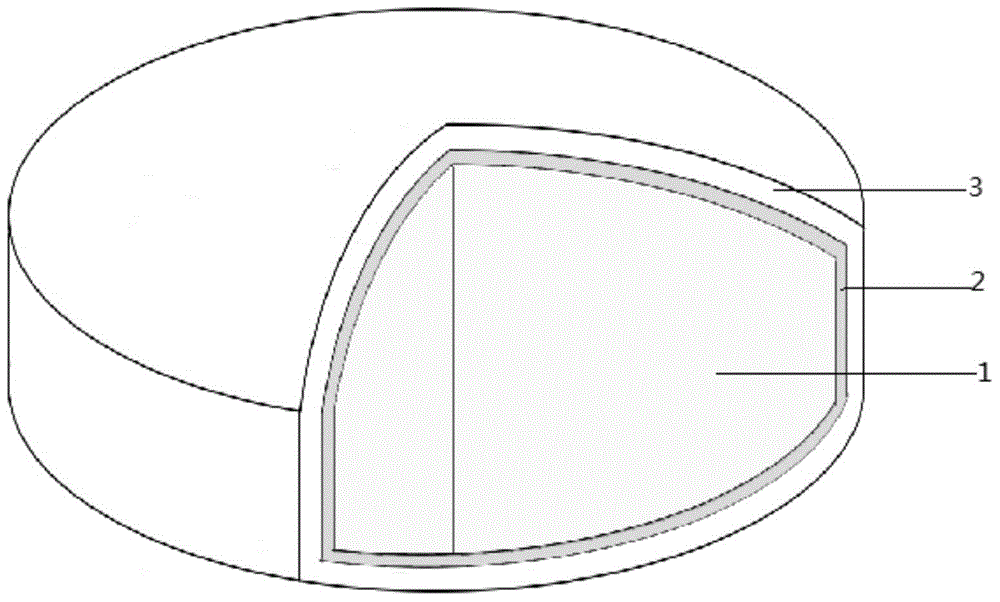
Image
Examples
Embodiment 1
[0098] The pretreatment of embodiment 1 proton pump inhibitor bulk drug
[0099] (1) Micronization treatment of omeprazole bulk drug
[0100] Weighed 2.5Kg of omeprazole crude drug through 80 mesh sieve, put it into QYF-100 jet mill and micronized to obtain 2.1Kg of micronized omeprazole, with a yield of 84.0%. Take the micronized omeprazole bulk drug sample and detect it with a Mastersizer 2000 Malvern laser particle size analyzer. The D50 is 1.9um and the D90 is 3.9um.
[0101] (2) Micronization treatment of esomeprazole magnesium bulk drug
[0102] Take by weighing 3Kg of the esomeprazole magnesium raw material medicine of 80 mesh sieves, put in QYF-100 jet mill and pulverize, obtain the esomeprazole magnesium of micronization 2.6Kg, the yield is 86.7%. Get the micronized esomeprazole magnesium crude drug sample, and detect it with a Mastersizer 2000 Malvern laser particle size analyzer, D50 is 2.2um, and D90 is 4.6um.
[0103] (3) micronization treatment of lansoprazole...
Embodiment 2
[0107] Preparation of Example 2 Proton Pump Inhibitor Enteric-coated Tablets
[0108] A. Preparation of Drug-Containing Tablet Cores
[0109] According to the prescription design in Table 1, take high-substituted hydroxypropyl cellulose and dissolve it in an appropriate amount of 75% ethanol aqueous solution, stir to dissolve completely, then add sodium hydroxide and Tween 80, stir to dissolve completely, and set aside; weigh according to the prescription design amount Proton pump inhibitor raw materials, mannitol, crospovidone XL-10, and magnesium oxide were passed through an 80-mesh sieve, and mixed in a one-step granulation mixer at a speed of 3 r / min for 10 minutes. Then, the above-mentioned binder was evenly added to the above-mentioned material, and the material was sheared at a high speed for 3 minutes, and the material was discharged. In the oscillating granulator, pass through a 24-mesh sieve. The wet granules of the proton pump inhibitor composition are placed in a...
Embodiment 3
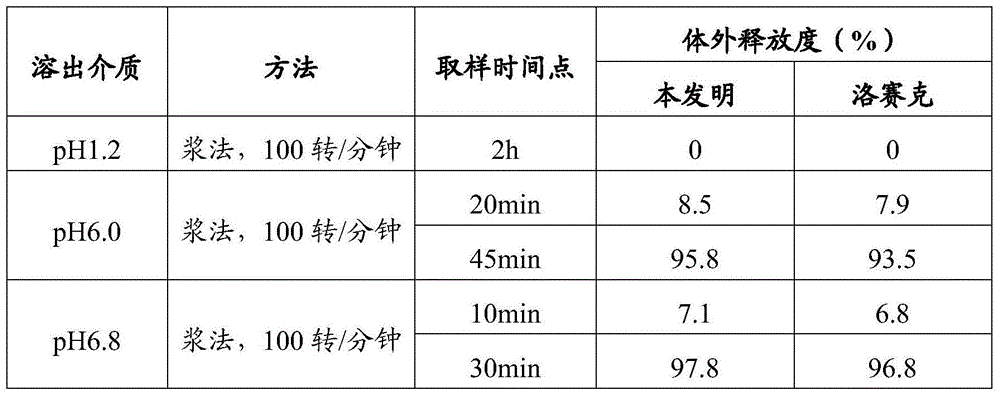
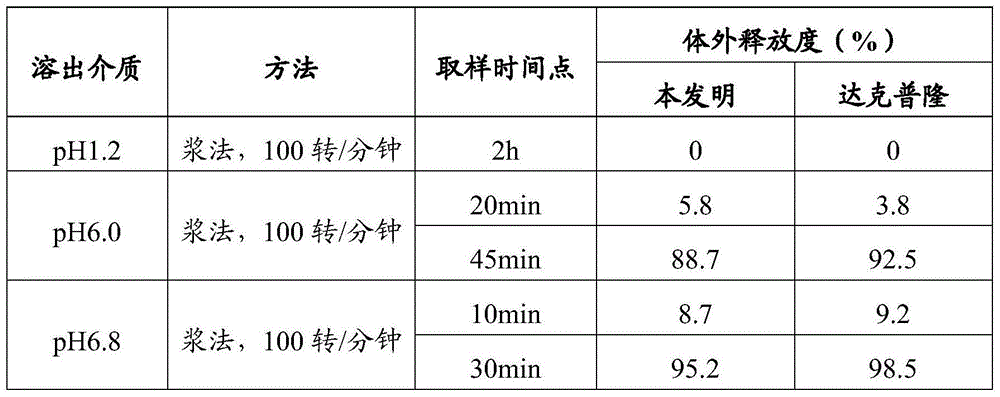
[0114] Example 3 In vitro release of omeprazole enteric-coated tablets prepared by the present invention in different dissolution media
[0115] Get the omeprazole enteric-coated tablet (10mg) that embodiment 2 prepares as test group object, according to release assay (appendix XD second method), adopt dissolution assay (appendix XC first method) device, with chlorine The hydrochloric acid solution of sodium chloride (take 1g of sodium chloride, add 3.5ml of hydrochloric acid, add water to 500ml) 500ml is the release medium, the rotating speed is 100 revolutions per minute, operate according to the law, after 120 minutes, immediately lift the rotating basket out of the liquid surface, None of the test pieces should have discoloration, cracks or disintegration. Immediately add 400ml of pH6.0 phosphate buffer solution or pH6.8 phosphate buffer solution preheated to 37°C to the operating container, keep the rotation speed unchanged, and continue to operate according to the law. A...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com