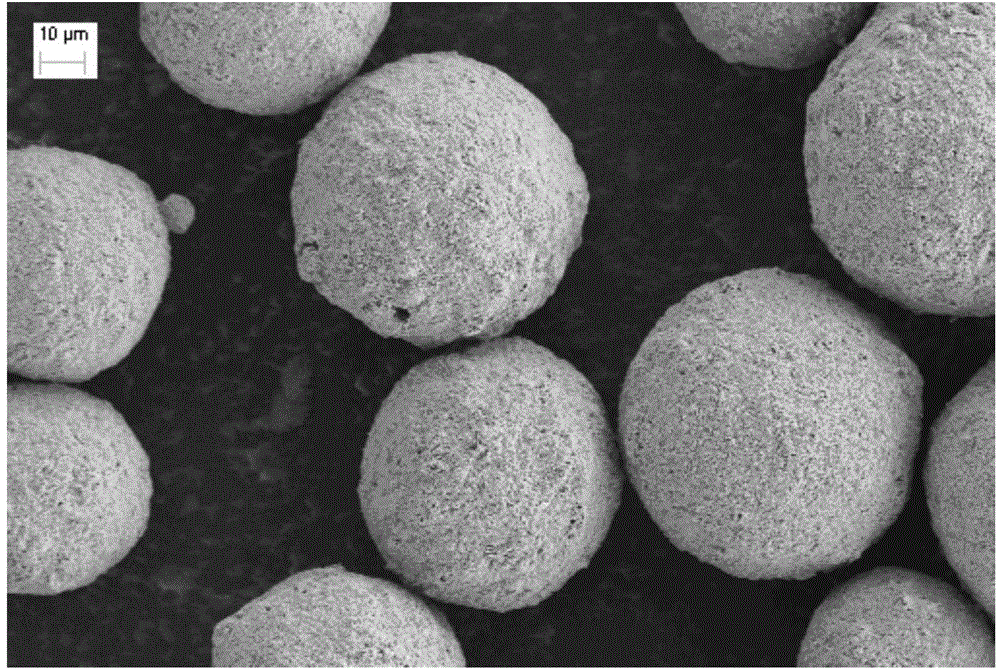
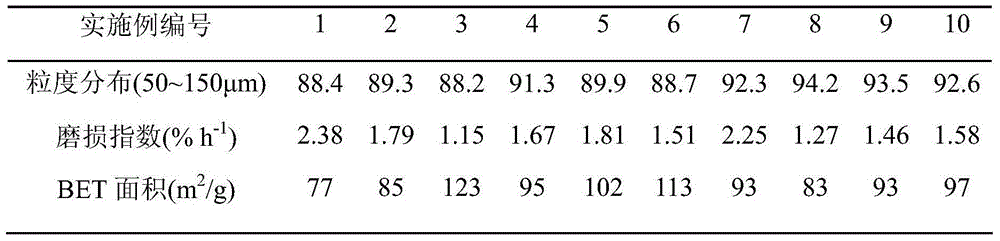
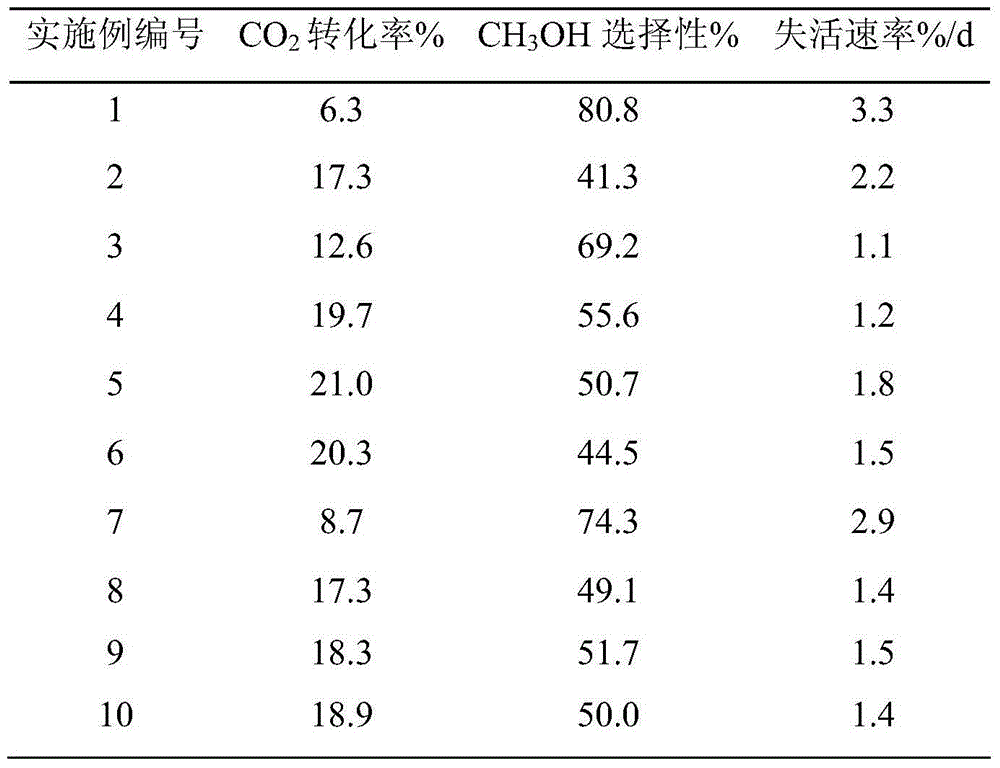
Catalyst for methanol synthesis via CO2 hydrogenation on slurry bed reactor, preparation method and application
A technology for synthesizing methanol and catalysts, which is applied in the preparation of organic compounds, chemical instruments and methods, and the preparation of hydroxyl compounds, etc. It can solve the problems of poor mechanical strength and wear resistance, no mention of catalyst wear resistance, and short life. , to achieve stable performance, high wear resistance and long life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The preparation of catalyst: according to CuO weight content in the final catalyst is 30wt% to take by weighing copper nitrate to be formulated into aqueous solution A (0.2mol / L) separately, according to ZnO, Al 2 o 3 , ZrO 2 In the final catalyst, the weight content is respectively 30wt%, 25wt%, and 10wt%. Take zinc nitrate, aluminum nitrate, zirconium nitrate, add deionized water and be mixed with mixed solution B (the concentration of zinc salt in mixed solution B is 0.1mol / L, the concentration of aluminum salt is 0.5mol / L, the concentration of zirconium salt is 0.025mol / L). Simultaneously, a mixed precipitation solution C of sodium hydroxide with a concentration of 0.5 mol / L and sodium carbonate of 1.0 mol / L was prepared. At 50°C, solution B and solution C are co-precipitated first, and after solution B is completely precipitated, solution A and solution C are co-precipitated, and the precipitation process needs to be fully stirred to maintain pH=12. After the p...
Embodiment 2
[0038] The preparation of catalyst: according to CuO weight content in the final catalyst is 50wt% to take by weighing copper nitrate to be formulated into aqueous solution A (0.5mol / L) separately, according to ZnO, Al 2 o 3 , ZrO 2 In the final catalyst, the weight content is respectively 20wt%, 10wt%, and 2wt%. Take zinc nitrate, aluminum nitrate, zirconium oxynitrate, add deionized water and be mixed with mixed solution B (the concentration of zinc salt in mixed solution B is 0.3mol / L, the concentration of aluminum salt is 0.1mol / L, the concentration of zirconium salt is 0.05mol / L). Preparation concentration is the sodium hydroxide of 1.2mol / L and the sodium carbonate mixed precipitation solution C of 0.2mol / L simultaneously. At 30°C, solution B and solution C are co-precipitated first, and after solution B is completely precipitated, solution A and solution C are co-precipitated. The precipitation process needs to be fully stirred to maintain pH=11. After the precipita...
Embodiment 3
[0041] The preparation of catalyst: according to CuO weight content in the final catalyst is 30wt% to take by weighing copper nitrate to be formulated into aqueous solution A (1.0mol / L) separately, according to ZnO, Al 2 o 3 , Y 2 o 3 In the final catalyst, the weight content is respectively 15wt%, 10wt%, 5wt% and takes zinc nitrate, aluminum nitrate, yttrium nitrate, adds deionized water and is mixed with mixed solution B (the concentration of zinc salt in mixed solution B is 0.5mol / L, the concentration of aluminum salt is 0.3mol / L, the concentration of yttrium salt is 0.01mol / L). Prepare concentration simultaneously and be the sodium hydroxide of 2.0mol / L and the sodium carbonate mixed precipitation solution C of 0.5mol / L. At 20°C, solution B and solution C are co-precipitated first, and after solution B is completely precipitated, solution A and solution C are co-precipitated, and the precipitation process needs to be fully stirred to maintain pH=10. After the precipit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com