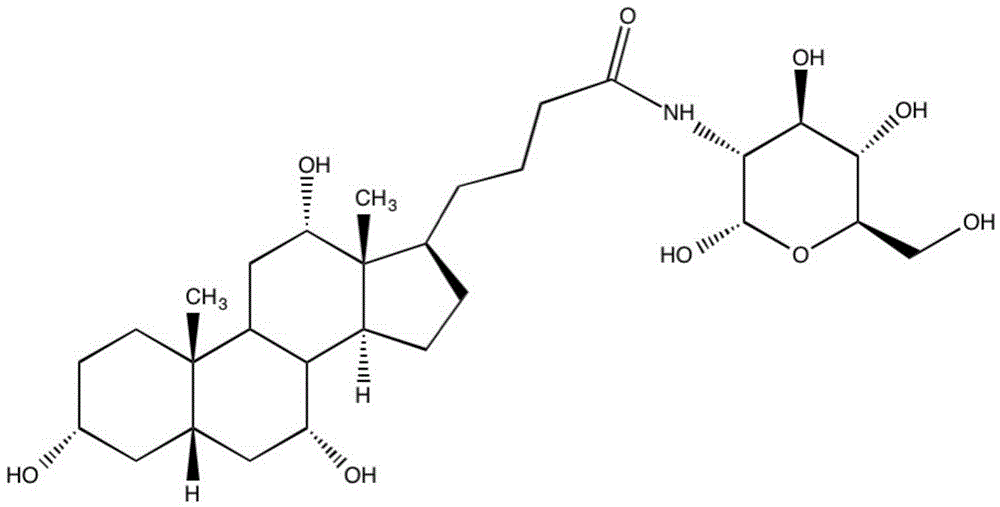
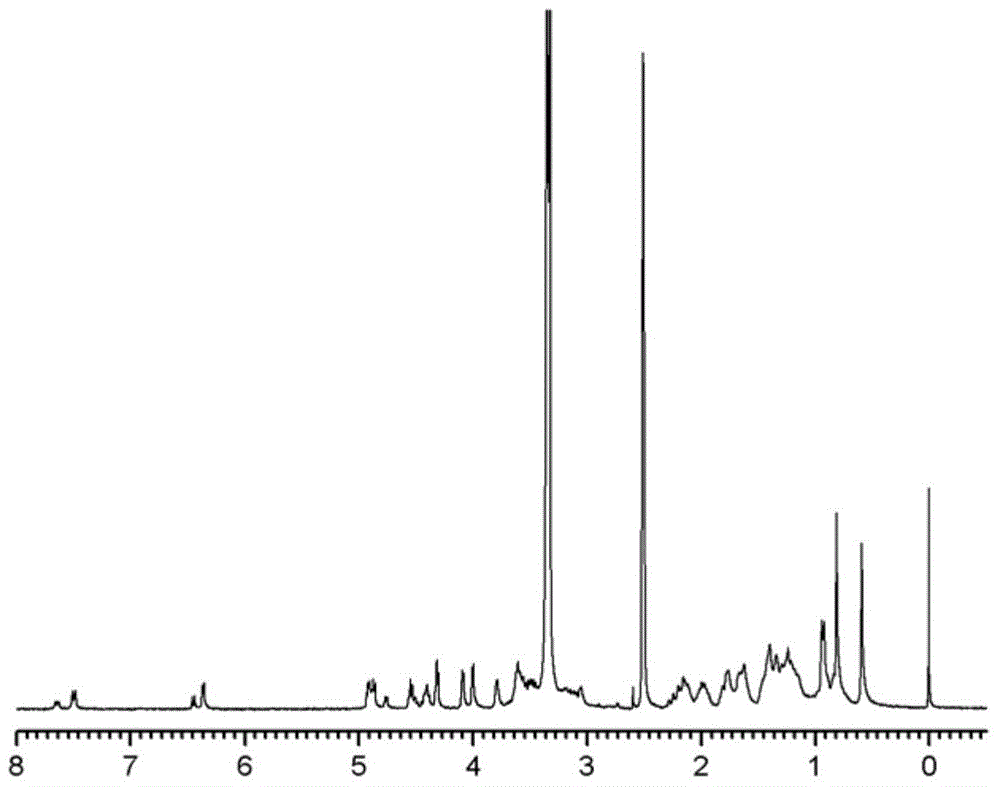
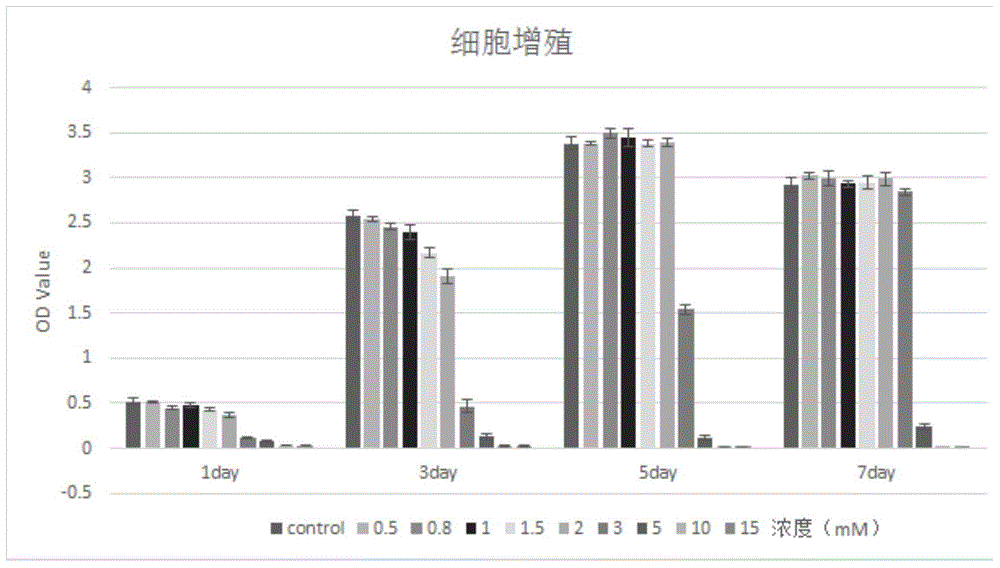
Cholic acid-modified glucosamine derivative and preparation method and application thereof
A technology of glucosamine and derivatives, which is applied in the field of bile acid modified glucosamine derivatives and its preparation, achieves the effects of high yield, convenient operation and unique biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Preparation of derivatives without polymerizable groups: cholic acid modified glucosamine derivatives, comprising the steps of:
[0036] Add 4.0858g (0.01mol) of cholic acid and 1.1509g (0.01mol) of N-hydroxysuccinimide into a 150ml three-necked flask, stir and dissolve in 30ml of tetrahydrofuran, and slowly add 3.0949g (0.015mol) of N , N'-dicyclohexylcarbodiimide in 10ml tetrahydrofuran solution, keep warm for 2h, return to room temperature, continue the reaction for 18h, then stop the reaction, filter the filtrate, and remove the solvent by rotary evaporation. Purified by silica gel column chromatography, the eluent was petroleum ether / ethyl acetate (1 / 4), to obtain 4.258 g of cholic acid active ester A, with a yield of 84.21%.
[0037] 2. Add 1563g (0.01mol) of glucosamine to a 100ml single-necked bottle, add 30ml of N,N-dimethylformamide / deionized water (2 / 1), then drop in 1.0119g (0.01mol) of triethyl Amine, stirred for 10 min, then added 5.057 g (0.01 mol) of ch...
Embodiment 2
[0043] Preparation of derivatives without polymerizable groups: cholic acid modified glucosamine derivatives, comprising the steps of:
[0044]Add 4.0858g (0.01mol) of cholic acid and 2.0269g (0.015mol) of 1-hydroxybenzotriazole into a 150ml three-necked flask, stir and dissolve in 30ml of tetrahydrofuran, and slowly add 3.8340g (0.02mol) of 10ml tetrahydrofuran solution of 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride, keep warm for 2h, return to room temperature, continue to react for 24h, then stop the reaction, take the filtrate by suction filtration, remove by rotary evaporation solvent. Purify by silica gel column chromatography, the eluent is petroleum ether / ethyl acetate (1 / 4), to obtain 3.833 g of cholic acid active ester B with a yield of 75.79%.
[0045] 3. Add 23445g (0.015mol) of glucosamine into a 100ml single-necked bottle, add 30ml of N,N-dimethylformamide / deionized water (2 / 1), then drop in 1.9386g (0.015mol) of N, N-diisopropylethylamine, stirr...
Embodiment 3
[0048] Preparation of derivatives with polymerizable groups: acryloylcholic acid modified glucosamine derivatives, comprising the following steps:
[0049] Add 4.0858g (0.01mol) of cholic acid and 1.1509g (0.01mol) of N-hydroxysuccinimide into a 150ml three-necked flask, stir and dissolve in 30ml of tetrahydrofuran, and slowly add 1.8915g (0.015mol) of N , N'-diisopropylcarbodiimide in 10ml tetrahydrofuran solution, keep warm for 2h, return to room temperature, continue to react for 18h, then stop the reaction, filter the filtrate, and remove the solvent by rotary evaporation. Purified by silica gel column chromatography, the eluent was petroleum ether / ethyl acetate (1 / 4), to obtain cholic acid active ester A, with a mass of 4.258 g and a yield of 84.21%.
[0050] Add 2.5283g (0.005mol) of bile acid active ester A, 1.012g (0.01mol) of triethylamine (without water) into a 100ml single-necked bottle, add 30ml of tetrahydrofuran (without water) at 0°C, and cool with dry ice To -...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com