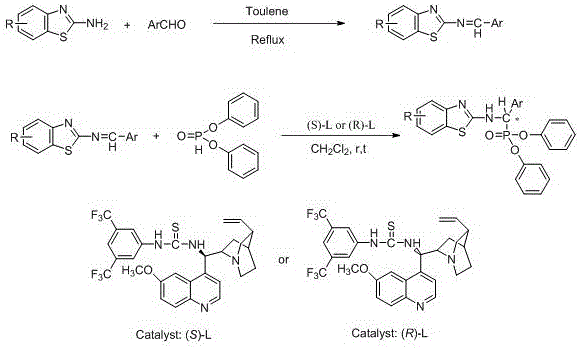
Chiral alpha-amino phosphonate ester compounds having anti-virus activity and containing benzothiazole heterocycle, preparation and applications thereof
An antiviral activity and aminophosphonate technology, which is applied to a class of chiral α-aminophosphonate compounds containing benzothiazole heterocycles with antiviral activity and the fields of preparation and application, can solve the problem of poor catalytic efficiency of catalysts. higher question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0097] Embodiment one, ( R )- O,O ’ -Diphenyl-1-(4-methylbenzo[ d ]thiazole-2-amino)-1-(phenyl)methylphosphonate (the compound number is ( R )-4a) Synthesis:
[0098] (1) Synthesis of (4-methylbenzothiazol-2-yl)-1-(phenyl)imine (3a):
[0099] Add 2-amino-4-methylbenzothiazole (1.64 g, 10 mmol), acetic acid (0.12 g, 20 mol %), toluene (50 mL) and benzaldehyde (1.02 g, 10 mmol ) into a 100 mL circle In a three-neck flask at the bottom, heat the system to reflux and divide water, cool to room temperature after reaction, evaporate the solvent under reduced pressure, and recrystallize petroleum ether to obtain a yellow solid imine with a yield of 56% and a melting point of 78-80 °C;
[0100] (2)( R )- O, O ’ -Diphenyl-1-(4-methylbenzo[ d Synthesis of ]thiazole-2-amino)-1-(phenyl)methylphosphonate
[0101] At room temperature, add 25 mg (0.1 mmol of phenyl-4-methylbenzothiazolimine, 6 mg (0.01 mmol ( S ) solid thiourea quinine catalyst, and 15 particles of freshly baked...
Embodiment 2
[0102] Embodiment two, ( R )- O, O ’ -Diphenyl-1-(4-methylbenzo[ d ]thiazole-2-amino)-1-(2-fluorophenyl)methylphosphonate (the compound number is ( R )-4b) Synthesis:
[0103](1) Synthesis of (4-methylbenzothiazol-2-yl)-1-(2-fluorophenyl)imine (3b):
[0104] Synthesize as in Example 1 (1) method and conditions. The difference is that 2-fluorobenzaldehyde (1.24 g, 10 mmol) was added, the reaction time was 10 h, the yield was 56%, and the melting point was 109-111°C.
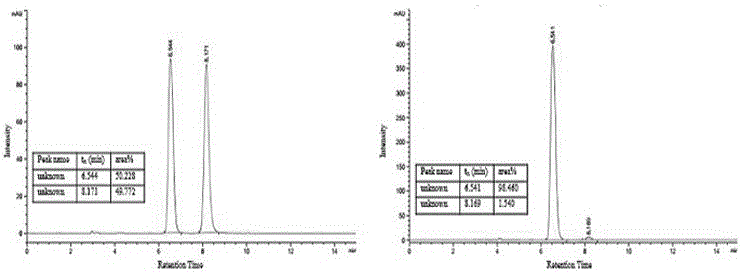
[0105] (2) Prepared according to the method and conditions of Example 1 (2). The difference is that 27 mg (0.1 mmol) of (2-fluorophenyl)-1-(4-methylbenzothiazole)imine was added and reacted for 1 hour to obtain 49 mg of the target compound with a yield of 98%. The target compound was tested for optical purity, the results are shown in image 3 ,Depend on image 3 It can be seen that the ee value is 97%. (Chromatographic conditions: using a chiral IA column, at room temperature, the detection wavelength ...
Embodiment 3
[0106] Embodiment three, ( R )- O, O ’ -Diphenyl-1-(4-methylbenzo[ d ]thiazole-2-amino)-1-(2-chlorophenyl)methylphosphonate (the compound number is ( R )-4c) Synthesis:
[0107] (1) Synthesis of (4-methylbenzothiazol-2-yl)-1-(2-chlorophenyl)imine (3c):
[0108] Synthesize as in Example 1 (1) method and conditions. The difference is that 2-chlorobenzaldehyde (1.40 g, 10 mmol) was added, the reaction time was 10 h, the yield was 48%, and the melting point was 137-138°C.
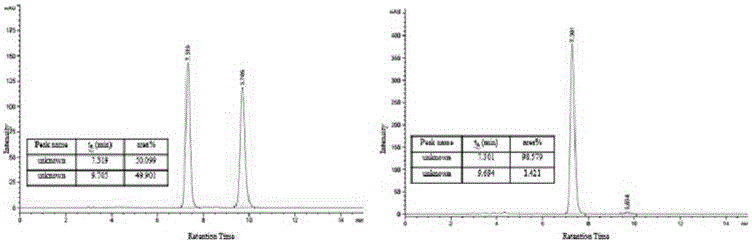
[0109] (2) Prepared according to the method and conditions of Example 1 (2). The difference is that 29 mg (0.1 mmol) of (2-chlorophenyl)-1-(4-methylbenzothiazole)imine was added and reacted for 1 hour to obtain 51 mg of the target compound with a yield of 98%. The target compound was tested for optical purity, the results are shown in Figure 4 ,Depend on Figure 4 It can be seen that the ee value is 97%. (Chromatographic conditions: using a chiral IA column, at room temperature, the detection wavele...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com