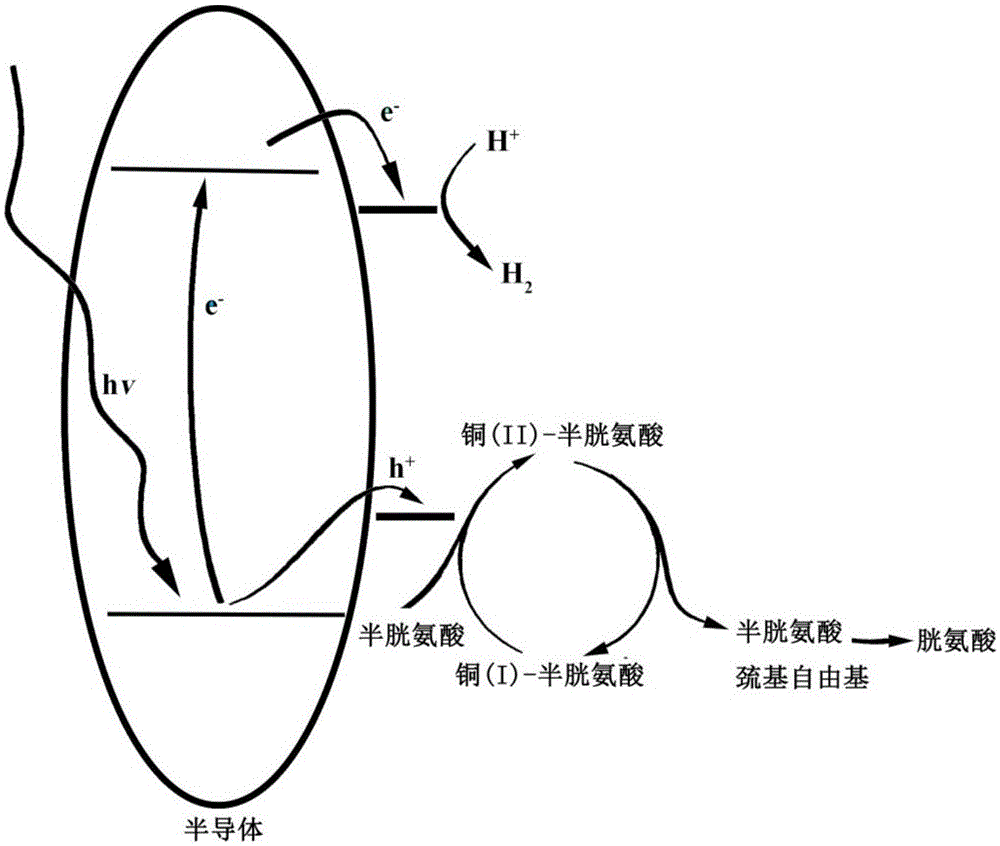
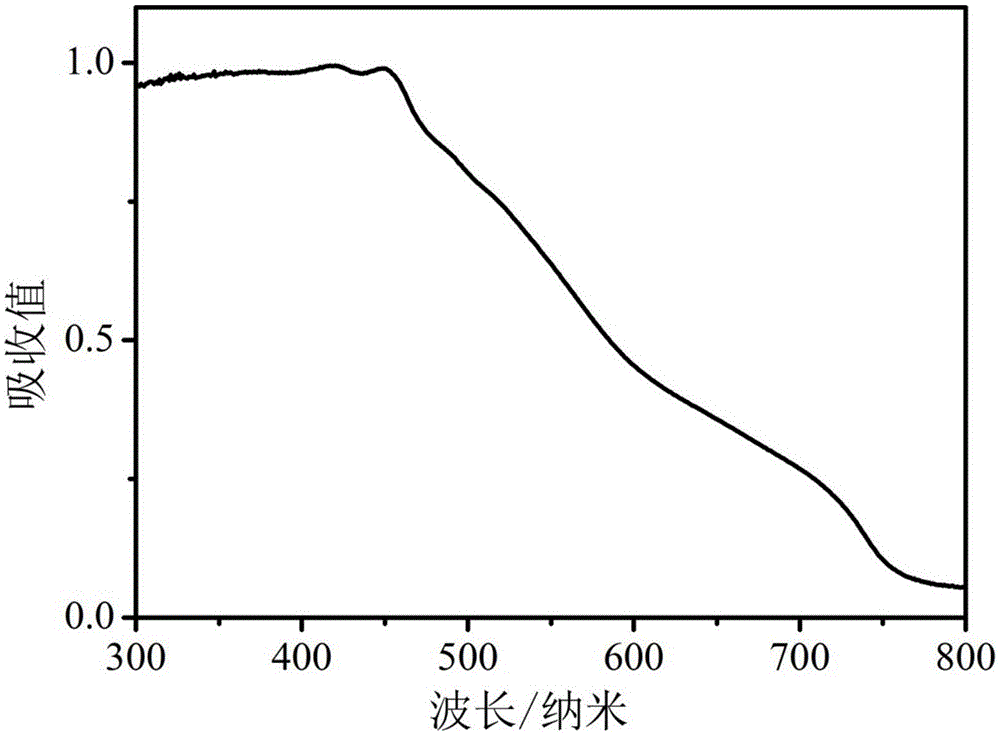
Visible light catalytic system with copper ion-thiol complex, preparation method, and hydrogen production method
A catalytic system and technology of mercaptan compounds, applied in organic compound/hydride/coordination complex catalysts, chemical instruments and methods, physical/chemical process catalysts, etc., to increase hydrogen production rate, increase hydrogen production rate, increase Effect of photocatalytic hydrogen production rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] A visible light photocatalytic system containing a copper ion-thiol complex and a hydrogen production method thereof, comprising the following steps:
[0042] Add 20mL of deionized water to a 60mL quartz tube, take 5mg of cadmium selenide sample into the quartz tube with deionized water, and ultrasonicate for 2-10 minutes to obtain suspension A; add 1.89mmol cysteine to suspension A Amino acid, sonicate for 2-5 minutes to obtain suspension B; then add 0.14 mg of copper ion-cysteine complex to suspension B, and sonicate for 3-5 minutes to obtain solution C; so far we have obtained copper Ion-thiol complex visible light photocatalytic system. Then add N into the solution D 2 Gas for 20-30 minutes, and then sealed with a rubber stopper; in an inert gas atmosphere, the reactor was irradiated with visible light (λ>400 nanometers) for several hours, and the hydrogen produced was characterized by gas chromatography.
Embodiment 2
[0044] First, the Pt-loaded Sn 2 Nb 2 o 7 , use 60mL size quartz tube to mix 10mg Sn 2 Nb 2 o 7 The powder was dispersed in 20 mL of aqueous solution containing 20 vol% methanol, and then 25 μL of H 2 PtCl 6 solution (10mmol / L). Subsequently, the air in the quartz tube was exhausted with nitrogen exhaust for 30 minutes, then sealed with a rubber stopper, and then the mixed solution was stirred for 30 minutes under a 500 watt high-pressure mercury lamp (XPA-7photochemical reactor, Nanjing Xujiang Machine-electronic Plant). The temperature of the reaction system is cooled with circulating water to maintain room temperature. Finally, the resulting Pt / Sn 2 Nb 2 o 7 The catalyst is separated by centrifugation.
[0045] Repeat embodiment 1, its difference is only to change the 5mg cadmium selenide catalyst that adds into 10mg Pt / Sn 2 Nb 2 o 7 , the same high photocatalytic hydrogen production rate system can be obtained.
Embodiment 3
[0047] Repeat Example 1, the only difference is that the addition of 0.47 μmol copper ion-cysteine complex is changed to 0.47 μmol copper nitrate, and the same high photocatalytic hydrogen production rate system can be obtained.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com