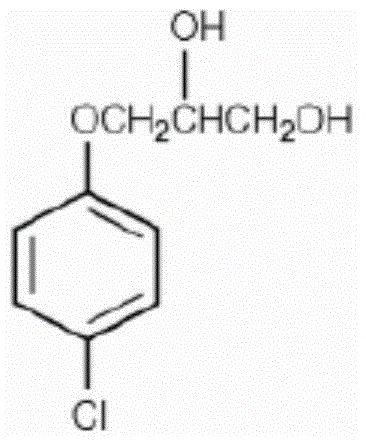
Synthetic method of chlorobenzene glyceryl ether
A technology of chlorphenesin and synthesis method, which is applied in the direction of ether preparation, ester reaction preparation of ether, ether separation/purification, etc., can solve the problems of strong corrosiveness of organic acids, reduced reaction yield, complex reaction raw materials, etc., and achieves Reduce the discharge of waste liquid, reduce the cost of raw materials, and improve the effect of purification yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
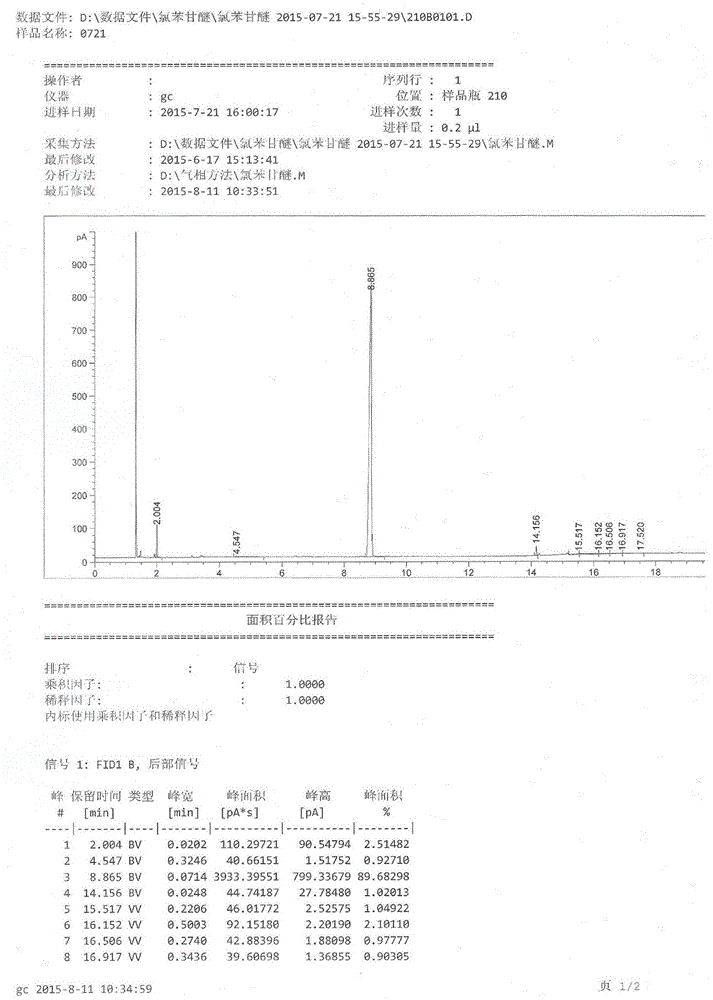
[0075] Take a 500ml three-necked flask, add 36.6g (content 99.5%) (0.33mol) of 3-chloro-1,2-propanediol, 16.6g (0.13mol) of p-chlorophenol, 34.0g of NaOH solution with a concentration of 18%, and heat the reaction system to 105°C, react for 1h. Add p-chlorophenol 9.0g (0.07mol), 18% NaOH solution 48.4g, 3-chloro-1,2-propanediol 4.5g (content 99.5%) (0.04mol), at this moment, in the system, p-chlorophenol and The molar ratio of the total amount of 3-chloro-1,2-propanediol added is 1:1.85, the molar ratio of the total added amount of p-chlorophenol and sodium hydroxide is 1:1.85, continue to react for 2 hours, cool down to 20°C, add dilute Hydrochloric acid to adjust the pH to 6.0. After standing for a period of time, the two phases were separated, and the lower organic phase was monitored by gas chromatography. The conversion rate was 89.3%, and then 150ml chloroform and 100ml water were added. The volume ratio of chloroform and water was 3:2, and the Stir in ice water, a whi...
Embodiment 2
[0077] Take a 500ml three-necked flask, add 36.6g (content 99.5%) (0.33mol) of 3-chloro-1,2-propanediol, 16.6g (0.13mol) of p-chlorophenol, 34.0g of NaOH solution with a concentration of 18%, tetrabutyl bromide Ammonium chloride 0.32g, the reaction system was heated to 105°C, and reacted for 1h. Add p-chlorophenol 9.0g (0.07mol), 18% NaOH solution 48.4g, 3-chloro-1,2-propanediol 4.5g (content 99.5%) (0.04mol), at this moment, in the system, p-chlorophenol and The mol ratio of 3-chloro-1,2-propanediol total addition is 1:1.85, and the mol ratio of p-chlorophenol and sodium hydroxide total addition is 1:1.85, and the mol ratio of p-chlorophenol and tetrabutylammonium bromide The molar ratio was 1:0.005, and the reaction was continued for 2 hours, the temperature was lowered to 20°C, and the pH value was adjusted to 6.0 by adding dilute hydrochloric acid. After standing for a period of time, two phases were separated, and the lower organic phase was monitored by gas chromatograp...
Embodiment 3
[0079] Take a 500ml three-necked flask, add 35.6g (content 99.5%) (0.32mol) of 3-chloro-1,2-propanediol, 16.6g (0.13mol) of p-chlorophenol, 73.2g of KOH solution with a concentration of 12%, tetrabutylsulfuric acid Ammonium hydrogen 0.5g, the reaction system was heated to 105°C, and reacted for 1h. Add p-chlorophenol 9.0g (0.07mol), 12% KOH solution 104.1g, 3-chloro-1,2-propanediol 4.3g (content 99.5%) (0.04mol), at this moment, p-chlorophenol and The molar ratio of the total amount of 3-chloro-1,2-propanediol added is 1:1.80, the molar ratio of the total added amount of p-chlorophenol and potassium hydroxide is 1:1.90, and the molar ratio of p-chlorophenol and tetrabutylammonium bisulfate The molar ratio was 1:0.007, and the reaction was continued for 2 hours, the temperature was lowered to 20°C, and the pH value was adjusted to 6.0 by adding dilute hydrochloric acid. After standing for a period of time, two phases were separated, and the lower organic phase was monitored by...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com