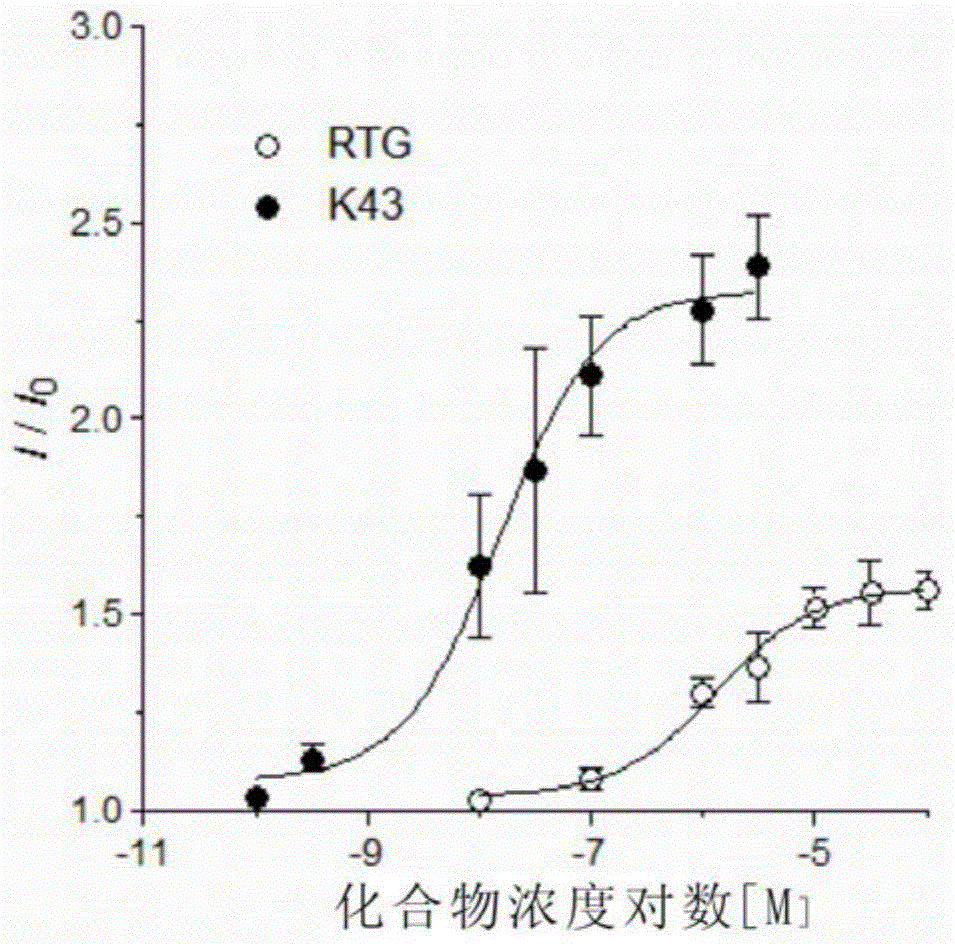
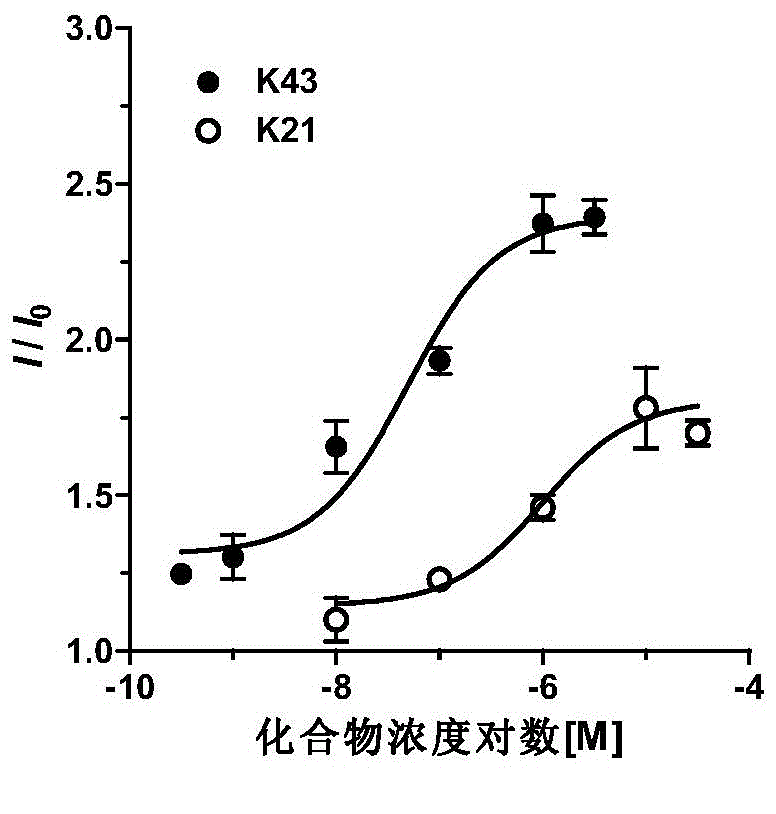
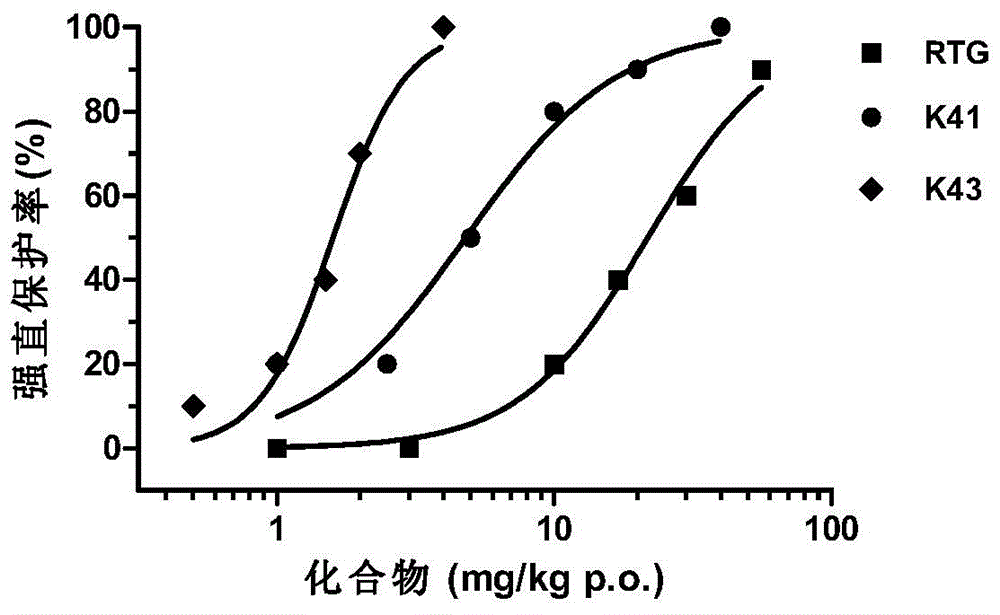
Novel KCNQ potassium channel agonist and preparation method and application thereof
A technology of general formula, C1-C4, applied to a new type of KCNQ potassium channel agonist, its preparation and use, can solve the problems of unclear mechanism of action, high neurotoxicity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0086] One, the preparation embodiment of compound
[0087] In the following preparation examples, nuclear magnetic resonance (NMR) is measured with a Mercury-Vx300M instrument produced by Varian (Varian), NMR calibration: δH7.26ppm (CDCl 3 ), 2.50ppm (DMSO-d 6 ), 3.15ppm (CD 3 OD); the reagents were mainly provided by Shanghai Chemical Reagent Company; the thin-layer chromatography (TLC) silica gel plate was produced by Shandong Yantai Huiyou Silica Gel Development Co., Ltd., model HSGF254; the normal phase column chromatography silica gel used for compound purification was Shandong Qingdao Ocean Chemical Factory Branch factory production, model zcx-11, 200-300 mesh.
preparation Embodiment 11
[0089] Preparation Example 1.1, Synthesis of 4-(N-p-fluorobenzyl-N-propargyl-amino)-2,6-dimethylphenylcarbamate methyl ester (K43)
[0090]
[0091] Compound 2,6-dimethylaniline K43-a (1.2g, 10mmol) was dissolved in dichloromethane (4mL) and pyridine (25mL), added p-toluenesulfonyl chloride (p-TsCl) (2.29g, 12mmol), reflux 6h. After cooling to room temperature, the reaction system was poured into 3M hydrochloric acid solution (20 mL), then dichloromethane (20 mL) was added, and the layers were separated. The obtained organic phase was washed twice with water (20 mL), concentrated, and the residue was recrystallized in ethanol. The product K43-b was obtained as a white solid (2.1 g, yield 76%). 1 H NMR (300MHz, CDCl 3 ): δ7.58(d, J=8.4Hz, 2H), 7.25(d, J=8.4Hz, 2H), 7.00-7.11(m, 3H), 5.96(s, 1H), 2.42(s, 3H) ,2.04(s,6H).
[0092] The obtained compound K43-b (2.10g, 7.6mmol) was dissolved in glacial acetic acid (AcOH) (40mL), water (40mL) and sodium nitrate (1.3g, 15.2mmol...
preparation Embodiment 12
[0099] Preparation Example 1.2, using operations similar to Preparation Example 1, the following compounds were prepared:
[0100]
[0101]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com