Green tea and carnitine capsule with function of reducing weight
A technology of green tea and carnitine, applied in the direction of capsule delivery, medical preparations containing active ingredients, organic active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Purchase and weigh 1.3 kilograms of L-carnitine tartrate, 0.4 kilograms of silicon dioxide, and 0.3 kilograms of magnesium stearate, for subsequent use;
[0025] Mix 9 kg of green tea hydrolyzate and 1.3 kg of L-carnitine tartrate evenly. The mixing time is not less than 30 minutes. The mixed material is granulated, dried, and granulated, and 0.4 kg of silicon dioxide is added to the dry granules. , 0.3 kilograms of magnesium stearate, mix homogeneously, then pack into capsule, polish, pack, radiation sterilize and make capsule preparation of the present invention.
[0026] Example 2:
Embodiment 2
[0028] Purchase and weigh 1.3 kilograms of L-carnitine tartrate, 0.4 kilograms of silicon dioxide, and 0.3 kilograms of magnesium stearate, for subsequent use;
[0029] Mix 9 kg of green tea hydrolyzate and 1.3 kg of L-carnitine tartrate evenly. The mixing time is not less than 30 minutes. The mixed material is granulated, dried, and granulated, and 0.4 kg of silicon dioxide is added to the dry granules. , 0.3 kilograms of magnesium stearate, mix homogeneously, then pack into capsule, polish, pack, radiation sterilize and make capsule preparation of the present invention.
[0030] Example 3
Embodiment 3
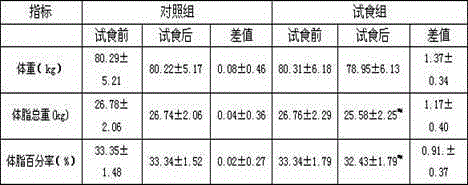
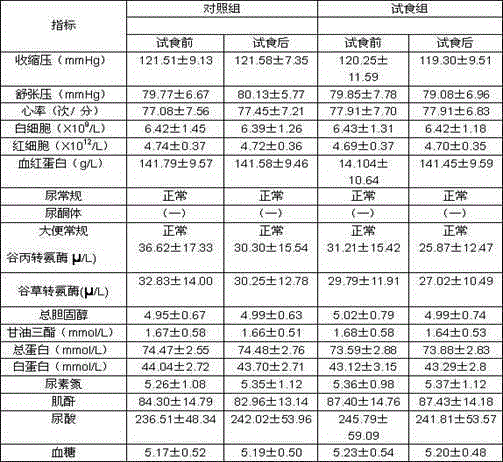
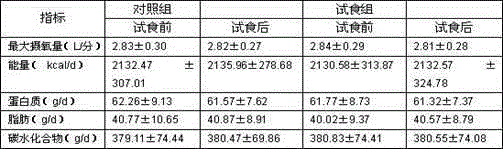
[0032] The weight loss function human body trial food test report provided by the present invention is as follows:
[0033] 1 Materials and methods
[0034] 1.1 sample: No. 1 and No. 2 green tea carnitine capsules of the present invention are all provided by the inventor, and the two are basically consistent on packaging, appearance, color and mouthfeel, wherein one is green tea carnitine capsules of the present invention, and the other is a placebo , The recommended oral dosage for human body is 3 times a day, 2 capsules each time.
[0035] 1.2 Subjects:
[0036] Subjects are not limited to gender, and those who are simply obese have no obvious heart, liver, gallbladder, kidney and other dysfunction after physical examination.
[0037] 1.2.1 Subject selection criteria:
[0038] Adult BMI ≥ 30, or voluntary subjects with a total fat percentage of > 25% for men and > 30% for women.
[0039] 1.2.2 Exclusion criteria:
[0040] 1.2.2.1 Patients with serious diseases such as h...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com