Gabapentin synthesis method
A synthetic method, gabapentin technology, applied in the field of gabapentin synthesis, can solve the problems of low yield and low product purity, achieve high yield, simple reaction route, and ensure the effect of total yield and purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
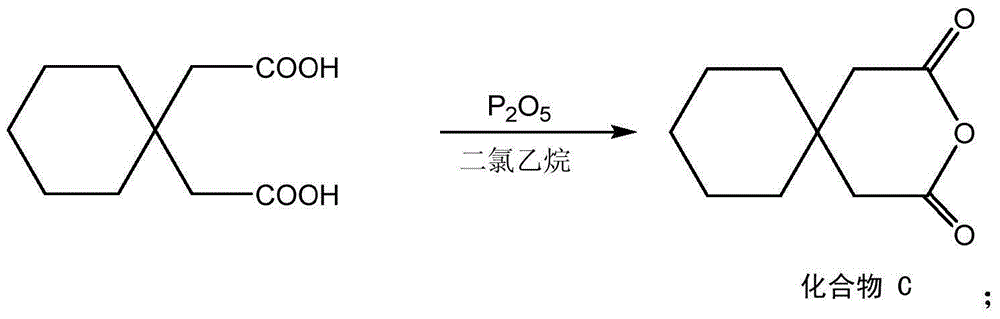
Examples
Embodiment 1
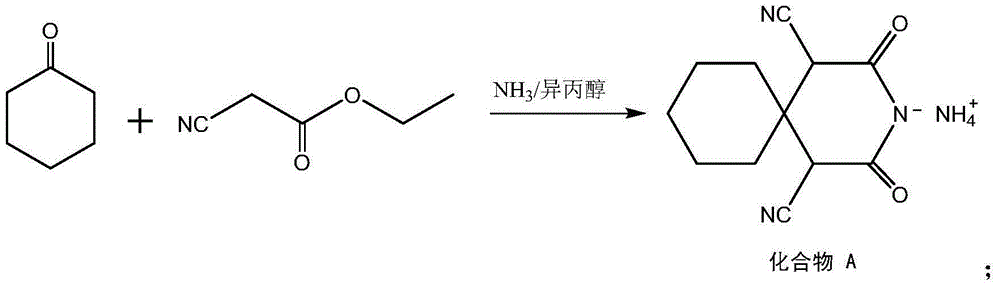
[0028] Put cyclohexanone in the reaction vessel, cool to -10°C, add 1.05 times the molarity of ethyl cyanoacetate in cyclohexanone, and add -10°C 4wt% liquid ammonia in anhydrous isopropanol solution at constant temperature while stirring , The amount of anhydrous isopropanol solution is 5 times that of cyclohexanone. At this temperature, let it stand for 9h, filter, wash with ether, and dry to obtain 2,4-dioxo-3-aza-spiro[5,5]undecane-1,5-dinitrile ammonium salt. Named as Compound A.
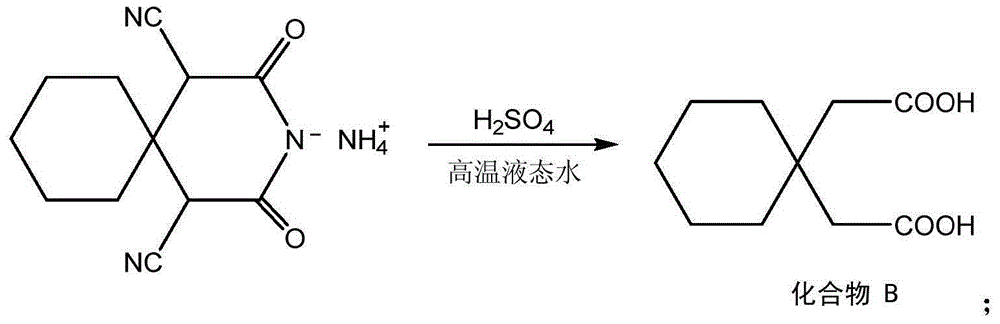
[0029] Put the above-mentioned compound A, 2 times the deionized water of compound A quality, the concentrated sulfuric acid (its consumption is so that the mass concentration of sulfuric acid in the reaction solution is 5% in the high-pressure reactor, adjust the pressure of the reactor so that the reaction solution The temperature is 205°C. Stop the reaction after the hydrolysis reaction for 3 hours, cool down, and cool down. Wash the solid twice with water, and recrystallize with a mixture ...
Embodiment 2
[0034] Put cyclohexanone in the reaction vessel, cool to -5°C, add ethyl cyanoacetate with 1.2 times the molarity of cyclohexanone, and add 16wt% liquid ammonia in anhydrous isopropanol solution at -5°C at constant temperature while stirring , The amount of anhydrous isopropanol solution is twice that of cyclohexanone. At this temperature, let it stand for 15h, filter, wash with ether, and dry to obtain 2,4-dioxo-3-aza-spiro[5,5]undecane-1,5-dinitrile ammonium salt. Named as Compound A.
[0035] Put the above-mentioned compound A, 10 times the deionized water of compound A quality, the vitriol oil (its consumption is so that the mass concentration of sulfuric acid in the reaction solution is 20% in the high-pressure reactor, adjust the pressure of the reactor so that the reaction solution The temperature is 235°C. Stop the reaction after the hydrolysis reaction for 1 hour, cool down, and cool down. Wash the solid twice with water, and recrystallize with a mixture of methanol:...
Embodiment 3
[0040] Put cyclohexanone in the reaction vessel, cool to -7°C, add 1.12 times the molarity of ethyl cyanoacetate in cyclohexanone, and add -7°C 10wt% liquid ammonia in anhydrous isopropanol solution at constant temperature while stirring , The amount of anhydrous isopropanol solution is 3.5 times that of cyclohexanone. At this temperature, let it stand for 12 hours, filter, wash with ether, and dry to obtain 2,4-dioxo-3-aza-spiro[5,5]undecane-1,5-dinitrile ammonium salt. Named as Compound A.
[0041] Put above-mentioned compound A, deionized water, concentrated sulfuric acid (its consumption is so that the mass concentration of sulfuric acid in the reaction solution is 12% in the reaction kettle of high pressure, 6 times of compound A quality, adjust the pressure of reaction kettle so that the reaction solution The temperature is 220°C. Stop the reaction after the hydrolysis reaction for 2 hours, cool down, and cool down. Wash the solid twice with water, and recrystallize wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com