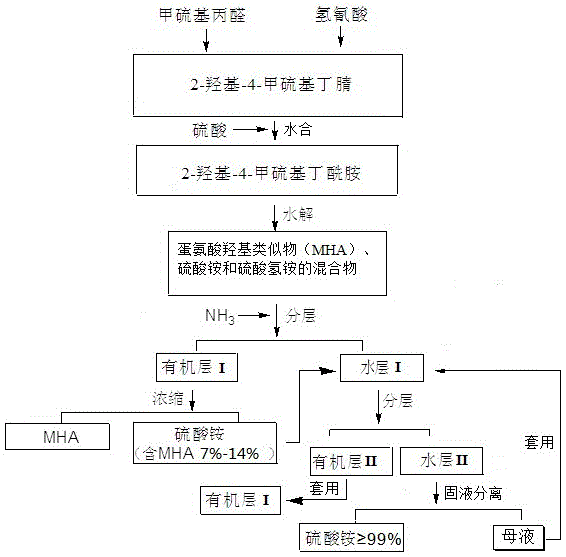
Separation and purification method for methionine hydroxy analogue synthesized through hydrolysis of cyanohydrins
A technology for the separation and purification of methionine hydroxyl groups, applied in the field of separation and purification of chemical products, can solve the problems of difficult drying, valueless, low yield, etc., to avoid recovery and loss, simple and easy method, low cost and energy consumption Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1, cyanohydrin hydrolysis method synthesis methionine hydroxyl analog
[0032] In a coolable reactor with a pH electrode and a thermometer, add 420.2g (4mol) of 99wt% methionaldehyde, adjust the pH value to 5.0~5.5 with aqueous sodium cyanide solution, and stir vigorously at 30°C. Cool, then slowly add 189g (4.2mol) of 60wt% hydrocyanic acid aqueous solution dropwise, control the reaction temperature not to exceed 40°C, and maintain the pH value of the reaction system at about 5.5 by continuously adding sodium cyanide aqueous solution during the dropping process . Hydrocyanic acid aqueous solution is added, remove cooling bath, 30 ℃ of stirring 2 hours, obtain the reaction solution 611.2g that contains 85.83wt% 2-hydroxyl-4-methylthiobutyronitrile, the reaction yield in terms of methylthiopropionaldehyde is 99.96%.
[0033] Add 339.4g (3.0mol) of 85wt% sulfuric acid aqueous solution into the reactor, and then slowly add the above-mentioned reaction soluti...
Embodiment 2
[0034] Example 2, Separation and purification of methionine hydroxyl analogs synthesized by cyanohydrin hydrolysis
[0035] Cool the hydrolyzed solution prepared in Example 1 to 40°C, and treat it with 2 mol of gaseous ammonia or ammonia water to convert the acidic ammonium bisulfate in the system into neutral ammonium sulfate, and ammonium sulfate solids are precipitated, and stand at 60°C for stratification , to obtain the organic layer I and an aqueous layer containing ammonium sulfate solids I , and its components and contents are shown in Table 1.
[0036] Table 1 Organic layer I and water layer I components and their contents
[0037]
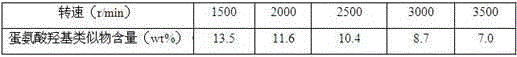
[0038] organic layer I Processing: the organic layer I Concentrate under reduced pressure until the moisture content is 3%, and ammonium sulfate solids are precipitated. Centrifuge at 60°C and different speeds (1500~3500r / min) for 20 minutes. The upper layer liquid separated by centrifugation is an oily yellow-brown liquid. Aft...
Embodiment 3
[0043] Example 3, Separation and purification of methionine hydroxyl analogs synthesized by cyanohydrin hydrolysis
[0044] Cool the hydrolyzate prepared in Example 1 to 40°C, and treat it with 2 mol of gaseous ammonia or ammonia water to convert the acidic ammonium bisulfate in the system into neutral ammonium sulfate, and ammonium sulfate solids are precipitated, and stand at 30°C for stratification , to obtain the organic layer I and an aqueous layer containing ammonium sulfate solids I .
[0045] organic layer I Processing: the organic layer I Concentrate under reduced pressure until the water content is 1%, and ammonium sulfate solids are precipitated. Centrifuge at 25°C and 1500r / min for 20 minutes. The upper liquid separated by centrifugation is an oily yellow-brown liquid. After testing, the ammonium sulfate content is 0.2 %, the content of methionine hydroxy analogs is 98.5%, and it is diluted with water until the content of methionine hydroxy analogs is 88%, th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com