Mycobacterium tuberculosis Rv 2991 recombinant protein, preparation method and application of mycobacterium tuberculosis Rv 2991 recombinant protein
A technology of Mycobacterium tuberculosis, rv2991, applied in the direction of botany equipment and methods, biochemical equipment and methods, applications, etc., can solve the problem of low diagnostic value, achieve high stability, reduce production costs, and high sensitivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Construction of embodiment 1 recombinant plasmid pET28a-Rv2991
[0038] (1) Target gene primer design
[0039] Rv2991-F (SEQ ID No: 3): GGAATTCCATATGGGAACCAAACAGCGCGC
[0040] Rv2991-R (SEQ ID No: 4): CCCAAGCTTGGCTACGGGGCGGTCGAGCCAC
[0041] The enzyme cutting sites are NdeI and HindIII respectively.
[0042] (2) PCR amplification, cloning and sequence determination of the target gene
[0043] Mycobacterium tuberculosis H 37Rv genomic DNA was used as a template, Rv2991-F and Rv2991-R were used as primers, and the Rv2991 protein gene was directly amplified by PCR using Taq enzyme (Bao Biological Engineering (Dalian) Co., Ltd.). PCR reaction conditions: pre-denaturation at 94°C for 5 minutes; (94°C, 30s; 58°C, 30s; 72°C, 40s) 35 cycles; extension at 72°C for 5 minutes; storage at 4°C. After the reaction, the target fragment was separated by 1% agarose gel electrophoresis, and then recovered with a DNA recovery kit (Invitrogen). Digested with NdeI and HindIII, and cl...
Embodiment 2
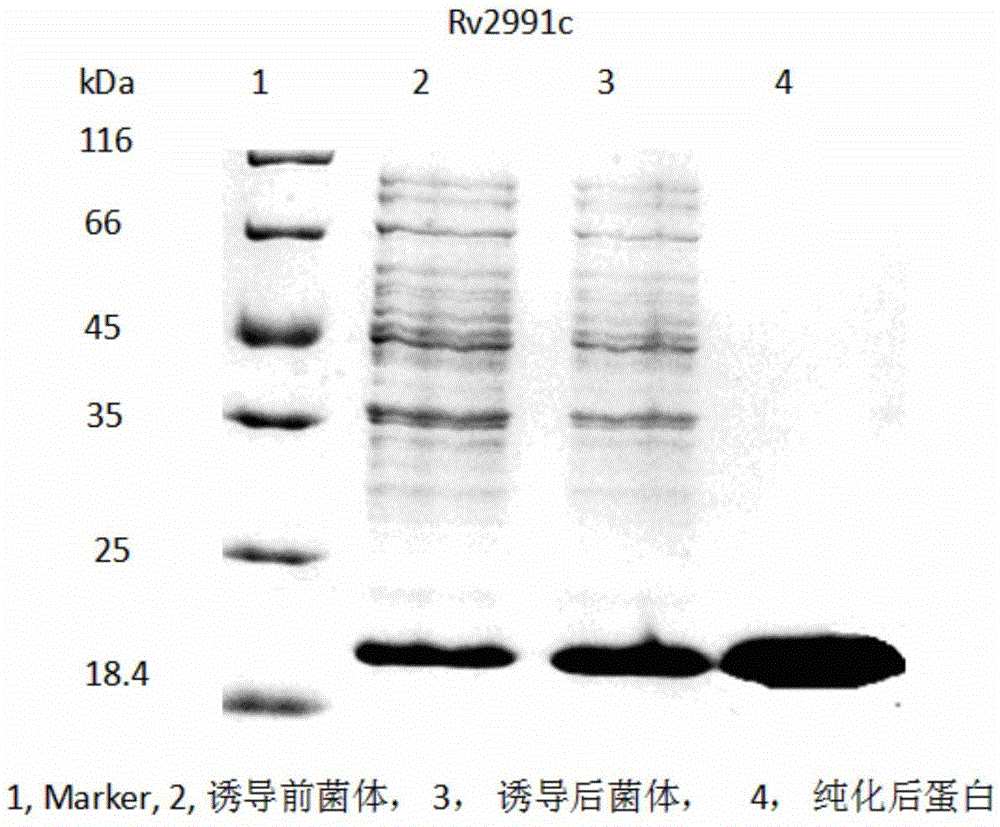
[0044] Example 2 Induced expression and purification of recombinant protein Rv2991
[0045] The eppdorf tube containing 100 μl of BL21(DE3) physS competent cells (TIANGEN) was immediately placed on ice from the -80°C freezer. After 3-5 minutes, wait for the liquid in the tube to melt, put 0.5 μl of the recombinant pET32a-Rv2991 plasmid with correct sequencing into competent cells, place it on ice for 45 minutes, place it in a water bath at 42°C, heat shock it for 90 seconds, and let it stand on ice for 3 minutes. Add 500 μl of preheated LB medium without antibiotics, shake at 37°C, 220rmp, and incubate for 45-60min. Take a certain amount and spread it on a solid LB medium plate containing kanamycin, dry it at room temperature and place it upside down in a 37°C incubator for overnight cultivation. Pick the clones, put them into LB liquid medium containing 50μg / ml kanamycin resistance, culture at 220rmp, 37°C to OD to about 0.6, add the final concentration of 10mMIPTG, 37°C for...
Embodiment 3
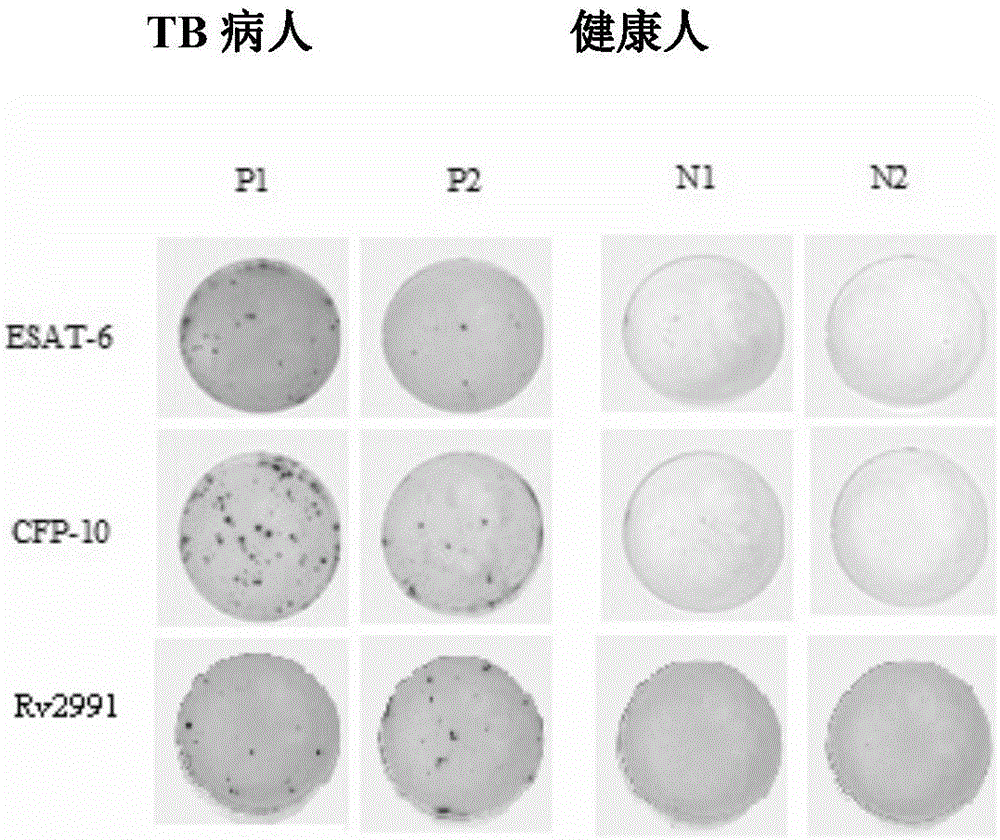
[0047] Example 3 Recombinant Rv2991 antigen is used as a detection reagent to detect clinically suspected tuberculosis patients
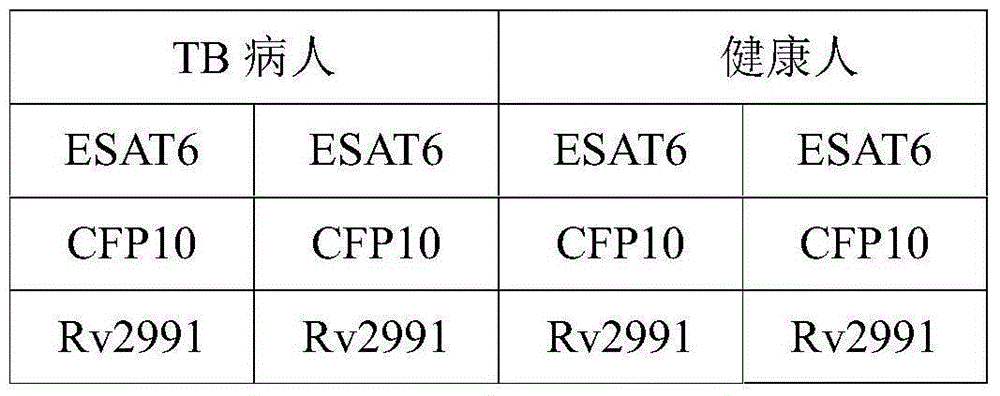
[0048] In this example, the recombinant Rv2991 antigen is used as a detection reagent to detect clinically suspected tuberculosis patients, and healthy people are used as a control group. At the same time, the experiment is carried out in groups with commonly used tuberculosis diagnostic antigens. The ELISPOT test design of specific antigens is shown in Table 1. The operation steps are as follows :
[0049] 1. Sample collection: Aseptically collect about 5ml of human peripheral venous blood into a heparin anticoagulant tube. After collection, the sample can be stored at room temperature, and should not be placed in a refrigerator or freezer; and marked;
[0050] 2. Separation, collection and counting of peripheral blood mononuclear lymphocytes:
[0051] a. Take 5ml of whole blood, add an equal volume of RTPMI-1640 serum-free culture medium, and mix...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com