Amphipathic oligomeric polypeptide drug conjugate
A technology of oligomeric polypeptides and conjugates, which is applied in the direction of drug combination, drug delivery, antipyretic drugs, etc., can solve the problems of insufficient drug preparations, and achieve the effects of rich variety, high yield, and convenient and quick preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: Preparation of amphiphilic oligomeric polypeptide drug conjugate (keto-VEVE) by valine and glutamic acid and anti-inflammatory drug ketoprofen
[0029] 1. Materials
[0030] Valine (V) protected with fluorenylmethyloxycarbonyl (Fmoc), glutamic acid (E) protected with fluorenylmethyloxycarbonyl (Fmoc) and tert-butyl (But), N,N'-diisopropyl Carbodiimide (DIC, 99%), 1-hydroxybenzotriazole (HOBT, 99%), 2-(1H-benzotriazole)-N,N,N',N'-tetramethylurea Hexafluorophosphate (HBTU, 99%), N,N-dimethylaminopyridine (DMAP, 99%), 9-fluorenylmethoxycarbonyl (Fmoc, 99%), N,N-diisopropylethylamine (DIEA, 99%), N,N-dimethylformamide (DMF, 99%), pyridine, piperidine, acetic anhydride, ninhydrin, king resin, acetonitrile, trifluoroacetic acid (TFA), methanol, 1 % Ammonia solution
[0031] 2. Preparation method
[0032] Utilize conventional solid-phase synthesis methods to synthesize oligomeric polypeptides, and then couple drugs. details as follows:
[0033] (1) First co...
Embodiment 2
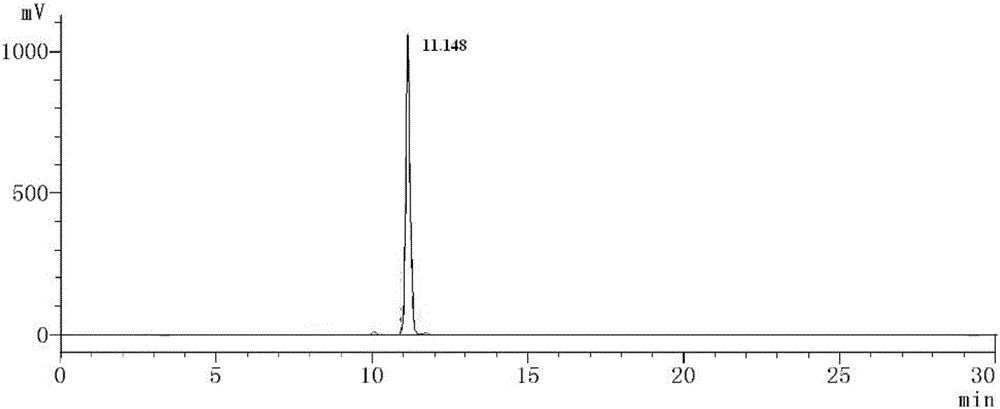
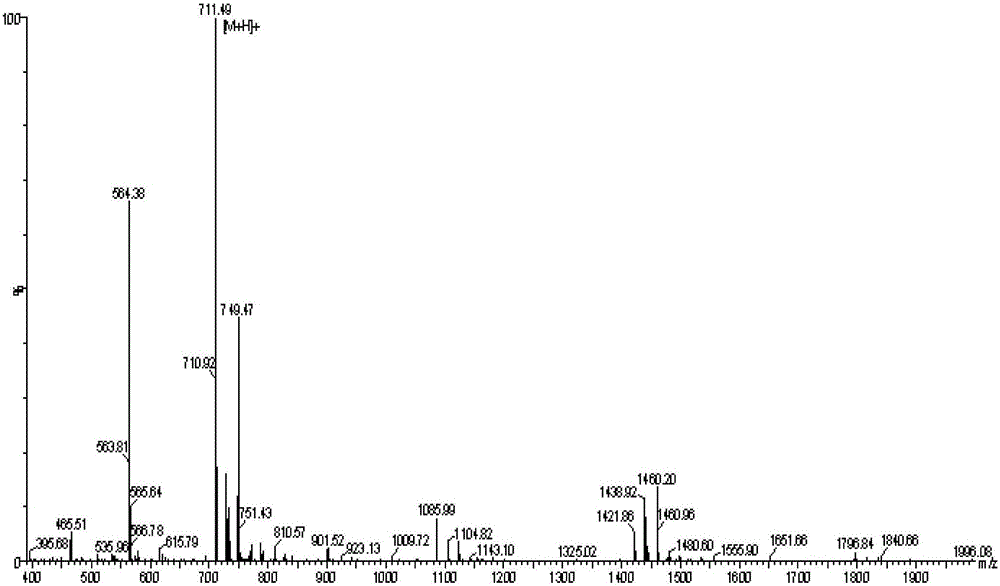
[0038] Example 2: High performance liquid chromatography and mass spectrometry detection of amphiphilic oligomeric polypeptide drug conjugate keto-VEVE
[0039] The keto-VEVE prepared in Example 1 is detected by high performance liquid chromatography (HPLC), see figure 1 ,Depend on figure 1 According to the peak area of the spectrum, the purity can be calculated to reach 97%. The keto-VEVE prepared in Example 1 is detected by mass spectrometry (MS), see figure 2 , indicating that its molecular weight is 710 is correct.
Embodiment 3
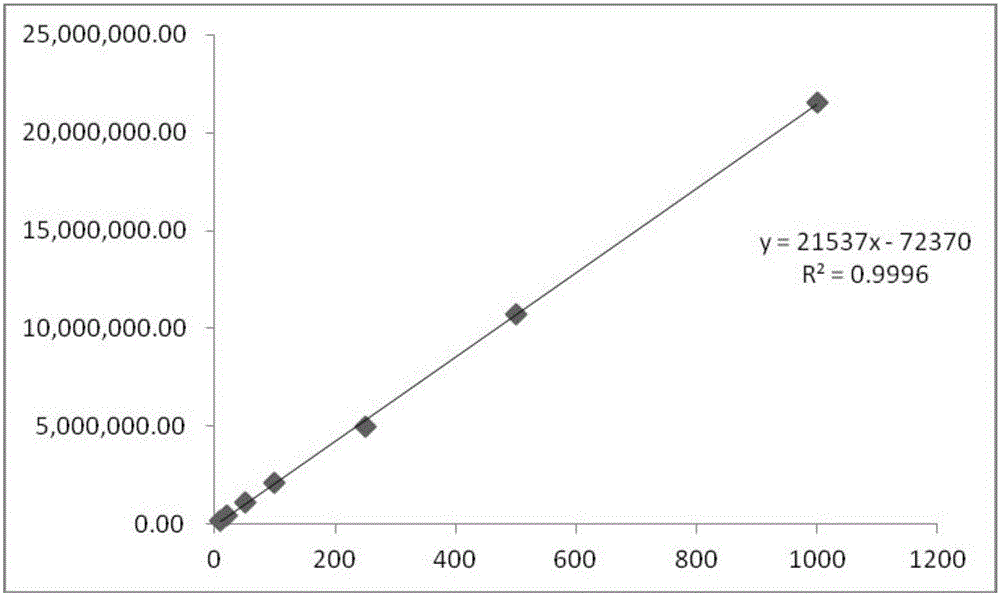
[0040] Embodiment 3: the establishment of the standard curve of keto-VEVE
[0041] Take the keto-VEVE prepared in Example 1 and make it into 1mg / ml mother liquor, dilute it step by step to 10, 20, 50, 100, 250, 500, 1000ug / ml, record the peak area in high performance liquid phase (HPLC), and draw a standard curve , see image 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com