Aryl-substituted amide compound, preparing method thereof, medicine composition comprising same, and application thereof
A technology for compounds and medicinal salts, applied in the preparation of organic compounds, drug combinations, preparation of carboxylic acid amides, etc., can solve the problems of lowering low-density lipoprotein, poor patient tolerance, large dosage, etc. The effect of promoting efflux
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0111] Example 1: Synthesis of representative compounds
[0112] (1) N-(2-(1H-indol-3-yl)ethyl)-2-aminobenzamide (CD1)
[0113] Tryptamine (purchased from Adamas, purity>99%) (0.80g, 5mmol) was added to a 50mL round-bottomed flask, 25mL N, N-dimethylformamide was added, followed by 2-aminobenzoic acid (0.68g , 5mmol), 1-ethyl-(3-dimethylaminopropyl) carbodiimide (EDCI) hydrochloride (all purchased from Beijing Coupling Technology Co., Ltd., purity>98%) (0.96g, 5mmol), React overnight at room temperature. Most of the solvent was evaporated under reduced pressure, 30 mL of water was added, and the mixture was extracted with ethyl acetate and dried with anhydrous sodium sulfate. The ethyl acetate extract was concentrated and eluted through a silica gel column with ethyl acetate: petroleum ether = 1:3 as the eluent to obtain 1.14g of pure CD1 (yield 82%), white solid, mp159-160℃ (literature value) 156-157°C). MS(ESIm / z)280.28(M+H) + , HRMS(ESIm / z)[M+H] + Theoretical formula C 17 H ...
Embodiment 2
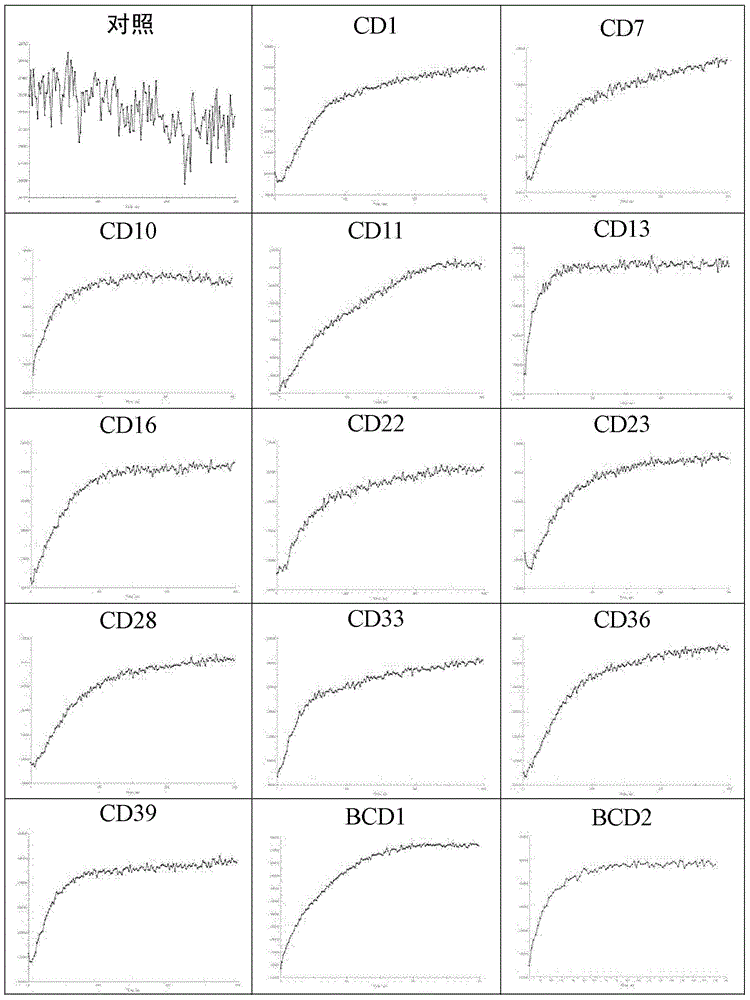
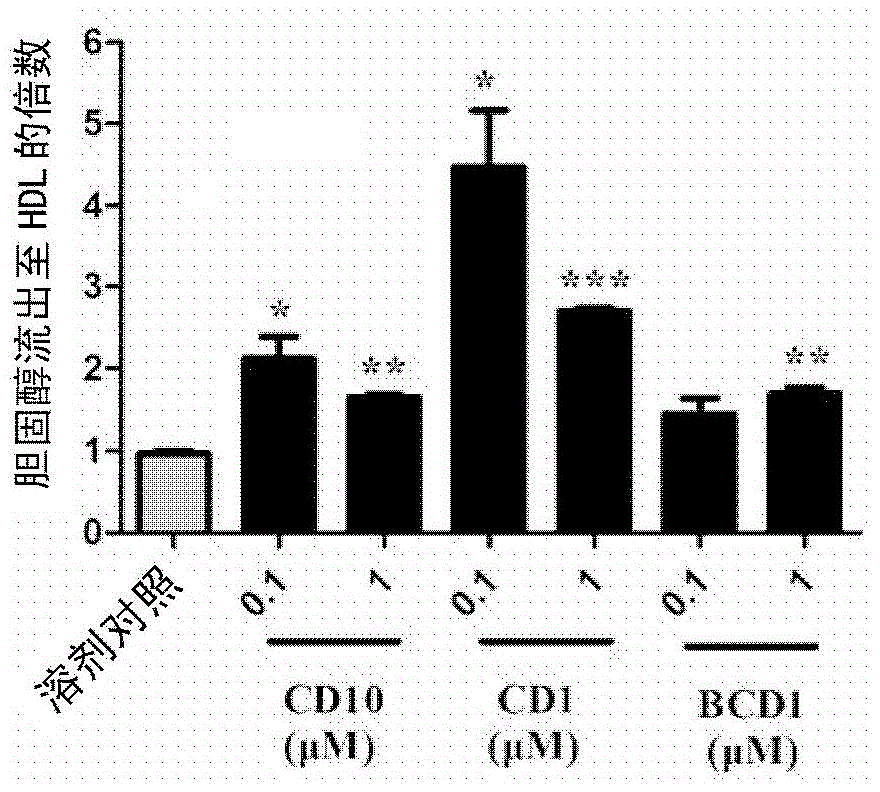
[0172] Example 2: The compound's activity on ABCA1 upregulator screening model
[0173] ABCA1p-LUCHepG2 cells were obtained from the National New Drug (Microbiology) Laboratory, Institute of Pharmaceutical Biotechnology, Chinese Academy of Medical Sciences. For the processing process of the cell, please refer to the article Identification of Up-regulators of HumanATP-binding Cassette Transporter Alvia High-throughput Screening of Synthetic and Natural Compound Library by Jie Gao et al. JBiomolScreen.2008, 13(7): 648 -656, and Gao Jie's doctoral dissertation "Construction and application of a new anti-atherosclerotic drug screening model targeting ABCA1".
[0174] Transfer ABCA1-LUCHepG2 cells to 5×10 4 Cells / well were seeded in a 96-well cell culture plate. After the cells adhered to the wall for about 6 hours, they were replaced with serum-free RPMI-1640 medium (Hyclone) (200 μl / well) containing a series of diluted concentrations of the compound of the present invention. Wells wi...
Embodiment 3
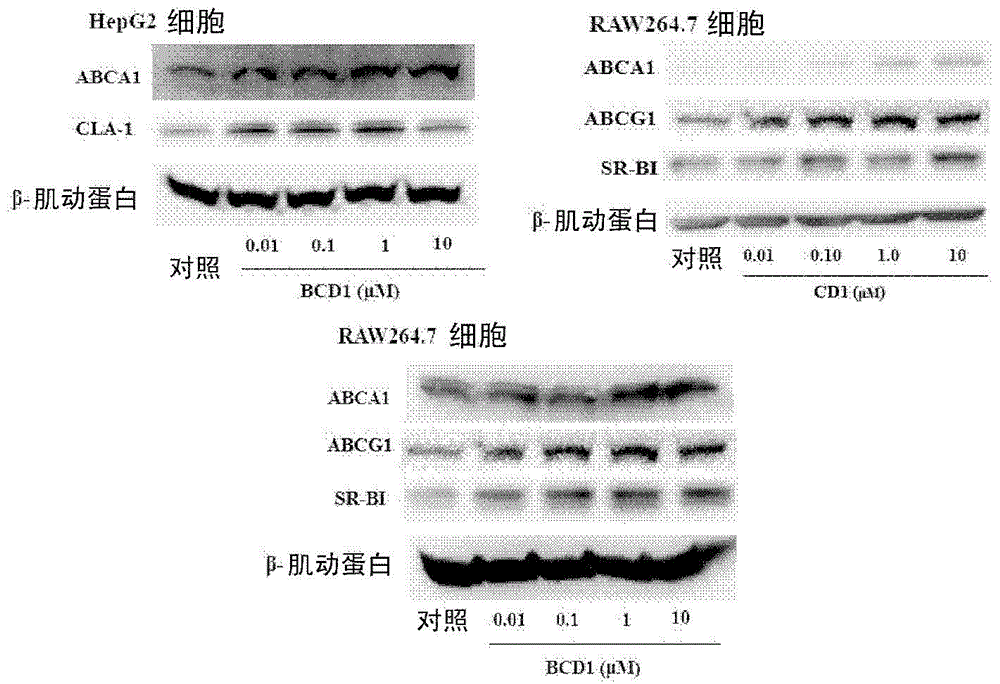
[0179] Example 3: The activity of the compound on the SR-BI / CLA1 upregulator screening model
[0180] CLAp-LUCHepG2 was obtained from the National Laboratory of New Drugs (Microbiology), Institute of Pharmaceutical Biotechnology, Chinese Academy of Medical Sciences. For the treatment process of this cell, please refer to the article IdentificationofNovelHumanHigh-DensityLipoproteinReceptorUp-regulatorsUsingaCell-BasedHigh-ThroughputScreeningAssay by Yuan Yang et al. JBiomolScreen2007, 12(2): 211-219, and Yang Yuan’s doctoral dissertation "Screening, Discovery and Related Activity Study of Microorganism-derived High Density Lipoprotein Receptor Upregulators".
[0181] Transfer CLap-LUCHepG2 cells to 5×10 4 Each cell / well was seeded in a 96-well cell culture plate, and after the cells adhered to the wall for about 6 hours, they were replaced with serum-free MEM-EBSS medium (200 μl / well) containing a series of diluted concentrations of the compound of the present invention. Keep the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com