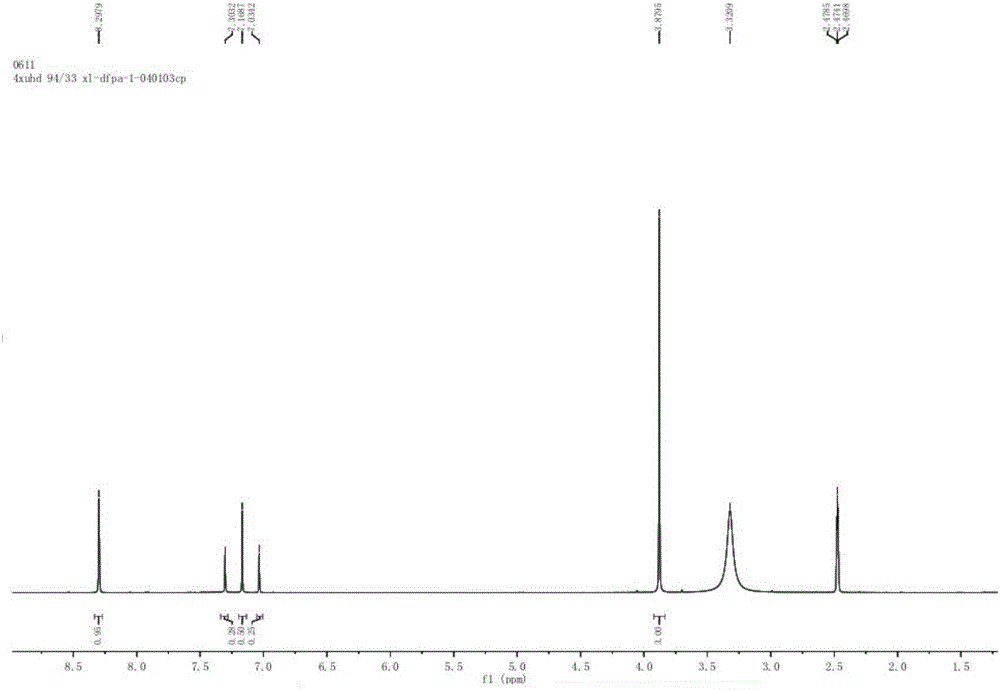
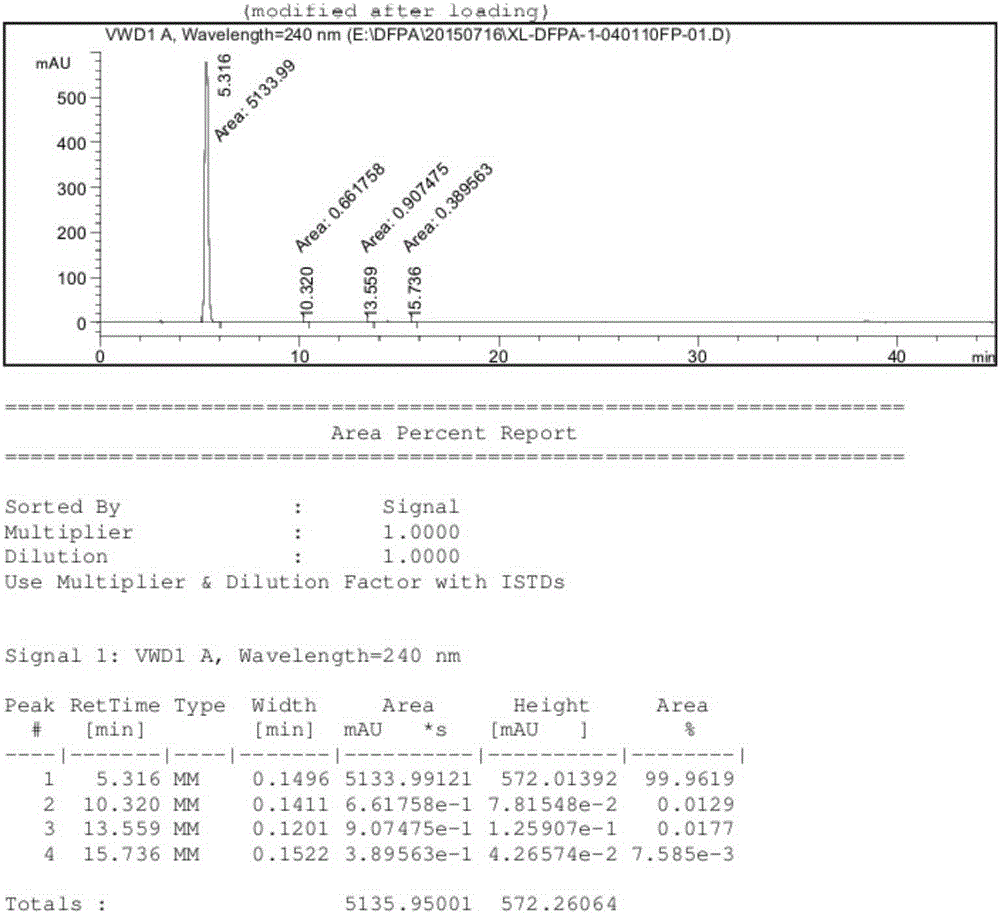
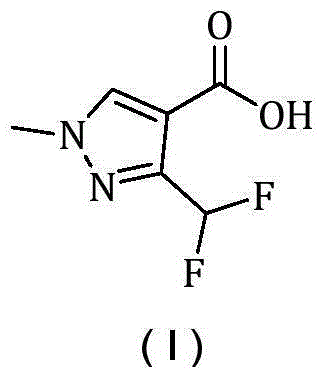
Synthesis method of 3-(difluoromethyl)-1-methyl-1H-pyrazole-4-carboxylic acid
A technology of difluoromethyl and pyrazole carboxylic acid, which is applied in the field of synthesis of fine chemical intermediates, can solve the problems of high industrialization cost, long route, and high price, and achieve the effects of low synthesis cost, high yield, and low raw material cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Step 1: Synthesis of ethyl 2-dichloroacetyl-3-(dimethylamino)acrylate (IV)
[0063] Put 100.12g of ethyl dichloroacetoacetate and 400g of toluene into a 2L reaction bottle, cool down to 0-10°C, add 20.56g of 60% sodium hydride in batches, control the internal temperature to less than 50°C, and keep for 1 hour after the addition ; Cool to 0-10°C, add 69.5g of [(CH 3 ) 2 N + =CHCl]Cl -
[0064] Soluble in 300g toluene solution. The reaction was maintained at 15-25°C for 2 hours. Pour the reaction material into ice water for quenching, and let stand to separate the toluene layer. After removing about 100 g of the solvent from the toluene layer under reduced pressure, 800 g of a toluene solution of ethyl 2-dichloroacetyl-3-(dimethylamino)acrylate (IV) was obtained (the nominal content was 13.40%, and the yield was 85%) ), used directly in the next step.
[0065] [(CH 3 ) 2 N + =CHCl]Cl - Prepare according to the following method: put 26.50g DMF and 300g toluene ...
Embodiment 2
[0080] Step 1: Synthesis of ethyl 2-dichloroacetyl-3-(dimethylamino)acrylate (IV)
[0081] In a 2L reaction flask, put 100g of ethyl dichloroacetoacetate and 400g of dichloromethane, cool down to 0-10°C, add 135g of [(CH3)2N + =CHCl][PO 2 Cl 2 ] - Dissolved in 300g dichloromethane solution. At 15-25°C, keep the reaction for 2 hours; slowly add 80g triethylamine dropwise at 0-5°C for about 1 hour; react at room temperature for 2 hours, add water and stir, separate the dichloromethane layer; Dissolved to obtain 135 g of crude product of ethyl 2-dichloroacetyl-3-(dimethylamino)acrylate (IV), which was directly used in the next step.
[0082] [(CH 3 ) 2 N + =CHCl][PO 2 Cl 2 ] - Prepare according to the following method: put 60.50g DMF and 300g dichloromethane into a 1L reaction bottle; stir and cool down to 0-10°C, add 85.55g phosphorus trichloride dropwise for about half an hour, and control the temperature 0-5 ℃, continue to stir for half an hour and set aside.
[00...
Embodiment 3
[0090] Step 1: Synthesis of ethyl 2-dichloroacetyl-3-(dimethylamino)acrylate (IV)
[0091] In a 2L reaction flask, put 100g of ethyl dichloroacetoacetate and 400g of methylene chloride into it, cool down to 0-10°C, add 135g of [(CH 3 ) 2 N + =CHCl][PO 2 Cl 2 ]- Dissolve in 300g toluene solution; slowly add 80g triethylamine dropwise at 0-5°C for about 1 hour; react at room temperature for 2 hours, add water and stir, separate the dichloromethane layer; desolvate under reduced pressure to obtain 2-dichloro The crude product of ethyl acetyl-3-(dimethylamino)acrylate (IV) was 150 g, the nominal yield was 76%, and it was directly used in the next step.
[0092] Step 2: Synthesis of ethyl 1-methyl-3-dichloromethyl-4-pyrazolecarboxylate (V)
[0093] In a 2L reaction flask, put 106.50g of 2-dichloroacetyl-3-(dimethylamino)ethyl acrylate (IV) and 400g of toluene, cool down to -60°C under nitrogen protection, and drop 19.62g of formazan Hydrazine (dissolved in 100 g of toluene); ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com