Cystamine diisocyanate monomer, cystamine diisocyanate monomer based polymers as well as preparation method and application of cystamine diisocyanate monomer
A cystamine diisocyanate and dihydroxy compound technology, which is applied in the preparation of hydrogenated polysulfides/polysulfides, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., can solve the application of unfavorable biomedical materials. and other problems, to achieve the effect of enriching varieties, reducing cumbersome steps and facilitating preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
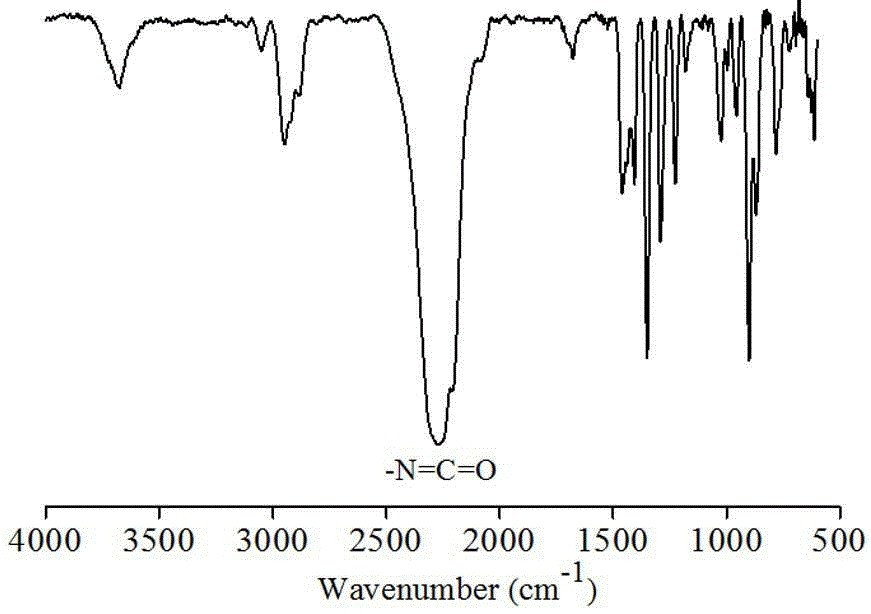
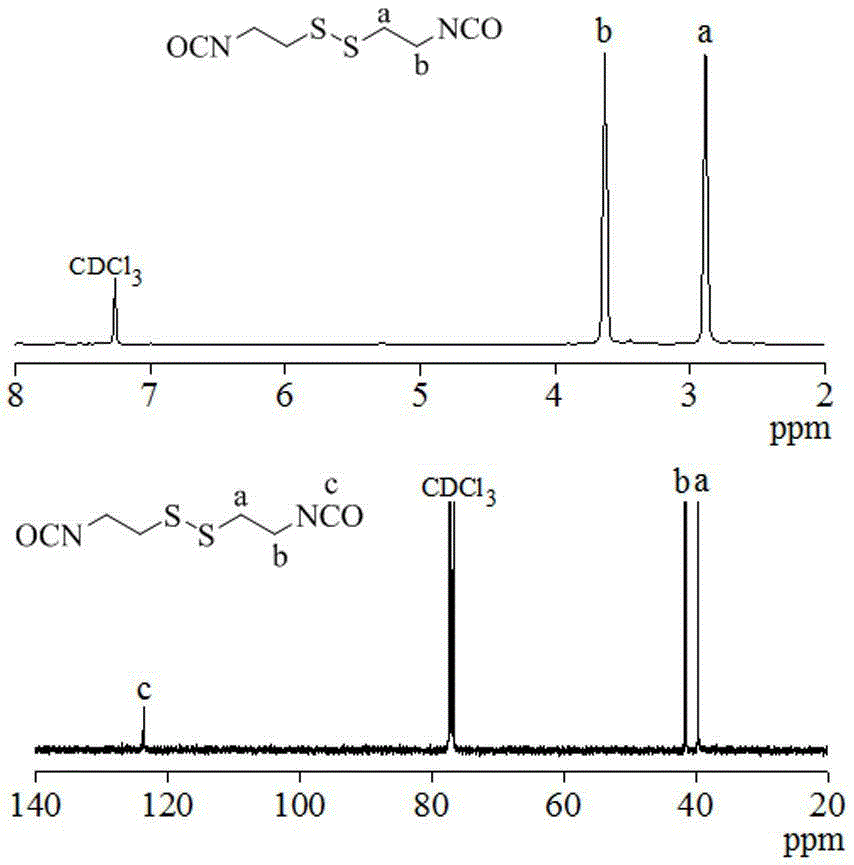
[0045] Synthesis of Cystamine Diisocyanate Monomer
[0046]
[0047] Under nitrogen atmosphere, cystamine dihydrochloride (11.25g, 0.05mol) and pyridine (23.73g, 0.3mol) were dissolved in 120mL of anhydrous dichloromethane, and poured into a 500mL three-neck flask. Dissolve triphosgene (8.91g, 0.03mol) in 40mL of anhydrous dichloromethane and add it to a 50mL constant pressure dropping funnel. Submerge the three-necked flask in an ice-salt bath, and when the temperature of the system drops to -10-15°C, under magnetic stirring, start to add triphosgene to the three-necked flask from a constant pressure dropping funnel to maintain the temperature of the system at - Reaction at 10°C for 6h.
[0048] After the reaction, extract three times with 0.1N glacial hydrochloric acid, take the organic phase, add anhydrous MgSO 4 Dry overnight, then filter with suction and concentrate by rotary evaporation to obtain a crude product, which is purified by distillation under reduced press...
Embodiment 2
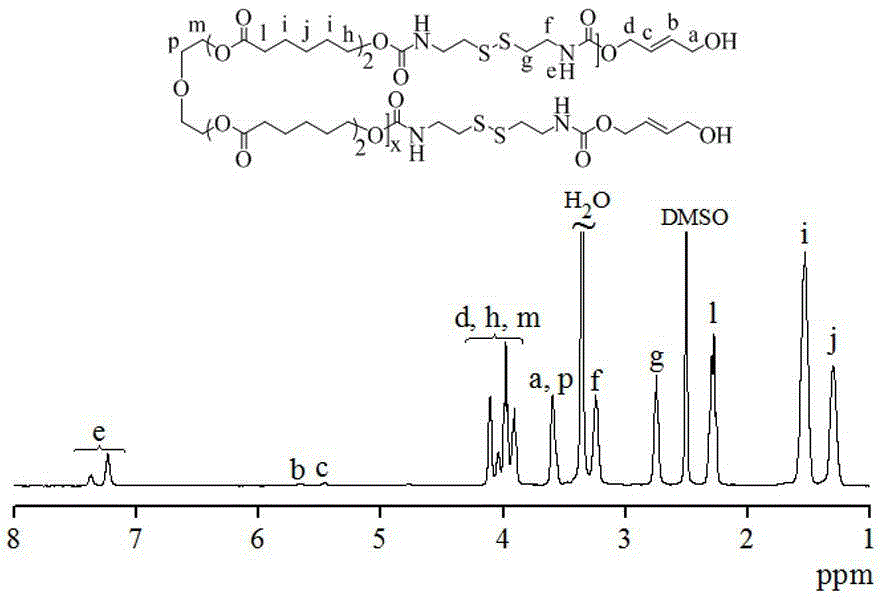
[0051] Cystamine diisocyanate monomer (CDI) undergoes polycondensation reaction with polycaprolactone (OCL) with hydroxyl groups at both ends to generate reductive and degradable polycaprolactone (SSPCL) containing disulfide bonds. The process is as follows:
[0052]
[0053] By changing the feed ratio of CDI and OCL, a series of SSPCLs with different molecular weights can be prepared. The raw material composition and GPC characterization results of the polymers are shown in Table 1.
[0054] Take the synthesis of SSPCL (Table 1, Polymer 1) as an example: under nitrogen protection, dissolve 0.210g cystamine diisocyanate in 1mL anhydrous DMF and then transfer to a closed reactor, then add 2.6mL to dissolve 0.530g OCL ( Mn=530g / mol) of anhydrous DMF, finally add a catalytic amount of dibutyltin dilaurate (7mg), and seal the reactor, put it in a 60°C oil bath for 24h, then add the end-capping agent 2-butene -1,4-diol continued to react for 12h. After the reaction, precipitate...
Embodiment 3
[0059] Cystamine diisocyanate monomer (CDI) undergoes polycondensation reaction with oligoethylene glycol (OEG) whose terminal group is a hydroxyl group to prepare reductively degradable polyethylene glycol (SSPEG) containing a disulfide bond. The process is as follows:
[0060]
[0061] A series of SSPEGs with different molecular weights can be prepared by changing the feed ratio of cystamine diisocyanate and oligoethylene glycol with hydroxyl-terminated groups. The raw material composition and GPC characterization results of the polymers are shown in Table 2.
[0062] Take the synthesis of SSPEG (Table 2, polymer 4) as an example: under the protection of nitrogen, dissolve 0.210g cystamine diisocyanate in 1mL anhydrous DMF and add to the closed reactor, then add 0.40g OEG (Mn=400g / mol) Dissolve in 2.1mL of anhydrous DMF and add to the closed reactor, finally add a catalytic amount of dibutyltin dilaurate (6mg), and seal the closed reactor, put it in an oil bath at 60°C, an...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Lc50 | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com