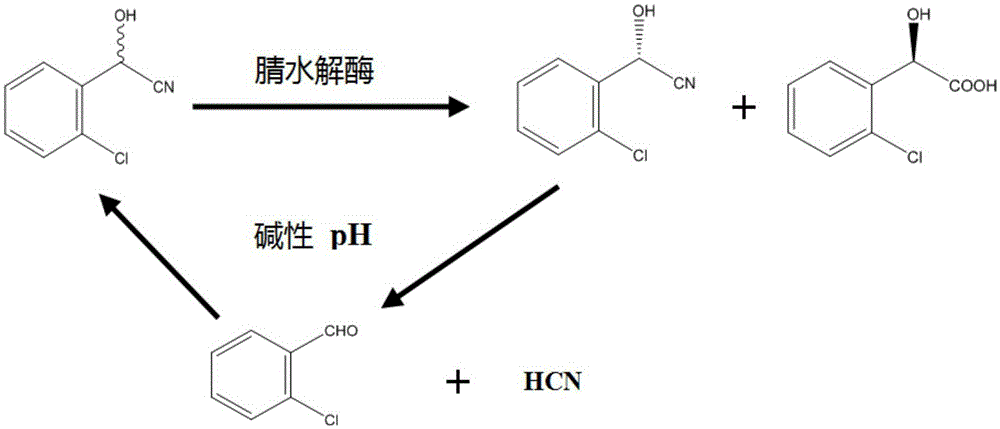
Method for preparing (R)-o-chloromandelic acid through enzyme and application of enzyme
A technology of o-chloromandelic acid and enzymatic preparation, applied in the field of preparation of pharmaceutical intermediate-o-chloromandelic acid, can solve the problems such as low purity of o-chloromandelic acid, high cost and complicated preparation method, and achieves low price, Increase the effect of correct folding and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1 prepares the E.coli engineering strain that has recombined nitrilase gene
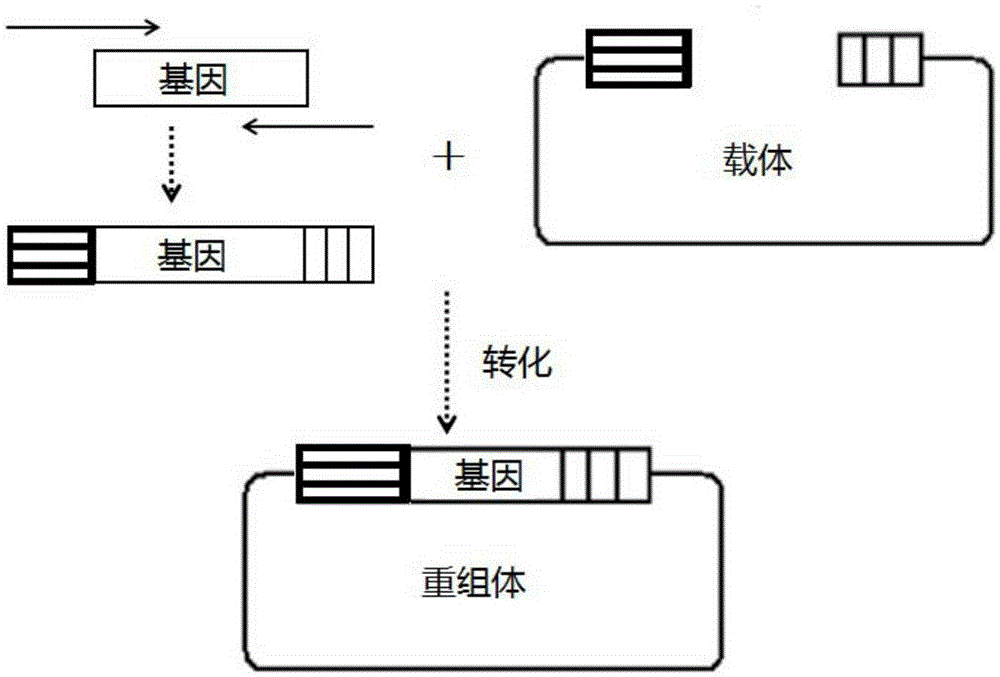
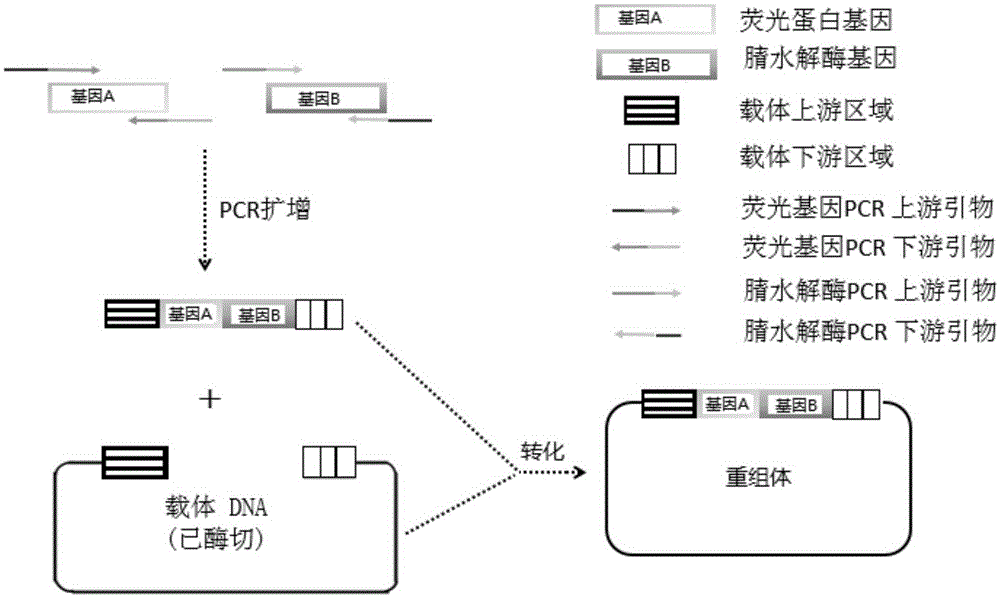
[0050] Prepare the E.coli engineering strain capable of expressing nitrilase by genetic engineering: the nitrilase gene and the fluorescent protein gene are recombined by an enzyme-free cloning method, and the process is as follows: image 3 As shown, enzyme-free cloning methods such as figure 1 shown. Then add DH5α Escherichia coli competent cells, carry out routine transformation coating plate, pick a single colony for PCR verification, inoculate the correct recombined colony in a small bacterial bottle and culture it overnight at 37°C and 200rpm on a shaker. The bacteria in the shake flask were collected, and the recombinant plasmid was obtained by extracting with a plasmid mini-extraction kit. The extracted plasmids were transformed into RosettaBlue (DE3) competent cells, and the single colonies obtained were respectively recorded as strain 1 # ~Strain 6 # , as a strain for ...
Embodiment 2
[0054] Embodiment 2 prepares SOB culture medium
[0055] The component content and preparation method of each liter of SOB medium are as follows: add 20 g of tryptone, 5 g of yeast extract, and 0.5 g of NaCl in 950 ml of deionized water, shake the container to completely dissolve the solute, add 10 ml of 250 mmol / L KCl Solution (dissolve 1.86g KCl in 100ml deionized water to make 250mmol / l KCl solution), adjust the pH value to 7.0 with 5mol / L KOH, adjust the volume to 1L with deionized water, and sterilize by autoclaving at 121°C for 30min. Before using this solution, add 5ml of sterilized 2mol / L MgCl 2 The solution is the SOB medium.
Embodiment 3
[0056] Embodiment 3 shake flask fermentation culture of recombinant strain
[0057] The bacterial strain 1 that embodiment 1 obtains # Inoculate into SOB liquid culture medium containing 50mg / ml kanamycin, shake the flask on a shaking table at 37°C and 200rpm, and when the OD600 of the bacteria reaches 0.5, move to a shaking table at 30°C overnight, and wait for the growth of the bacteria to Take it out at the end of the plateau phase, and collect resting cells by centrifugation (6000rpm, 5min) at 4°C, and record it as resting cells 1 # , the mass of wet bacteria reaches 4g / L.
[0058] strain 2 # , strain 3 # The same fermentation and culture operation as the same, the obtained resting cells were recorded as resting cells 2 # , resting cells 3 # . resting cell 1 # ~ resting cell 3 # The wet thalline mass is shown in Table 2.
[0059] Table 2
[0060] resting cell number
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com