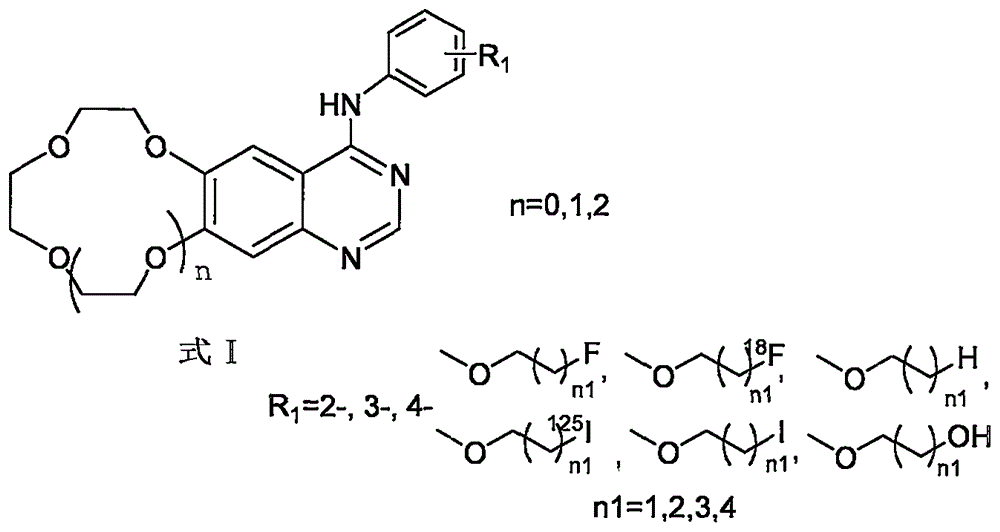
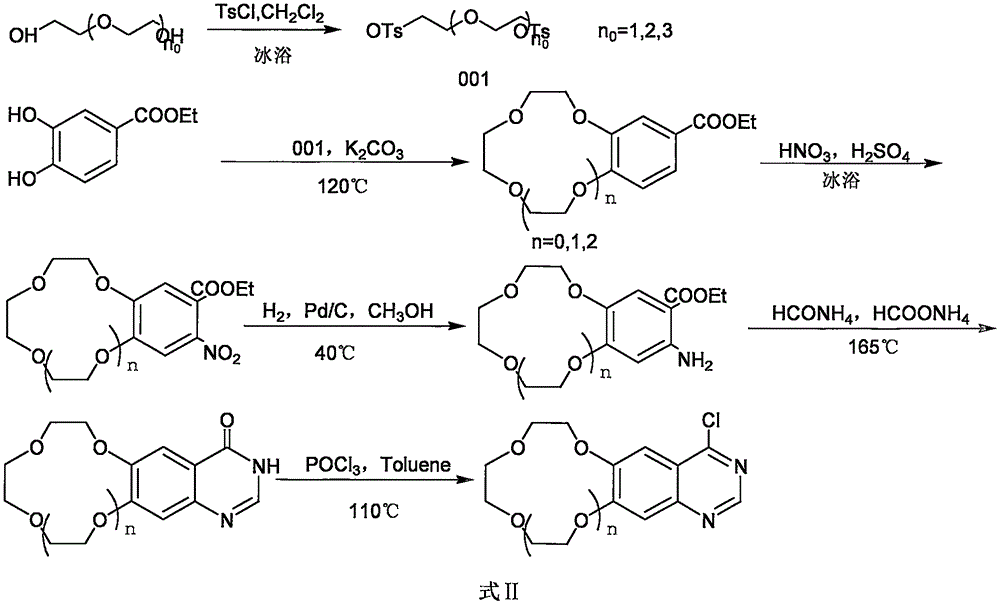
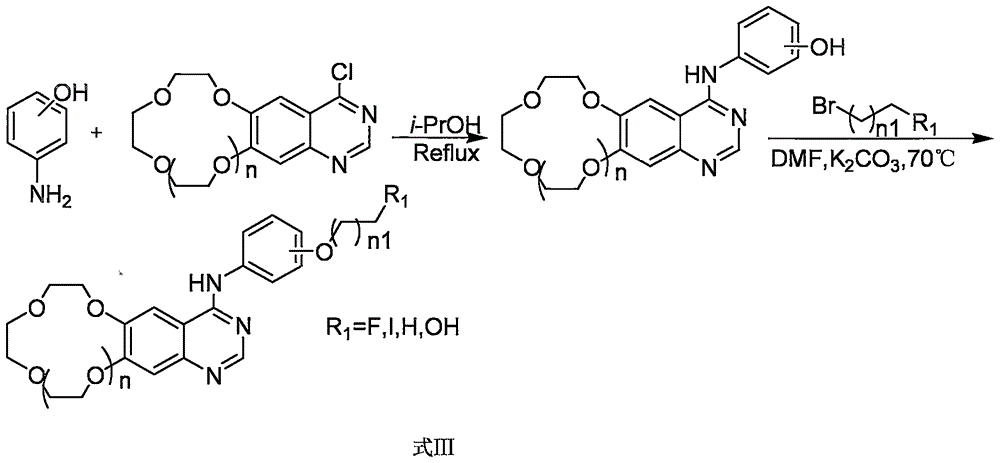
Crown ether cyclic quinazoline compound, preparation method therefor and application thereof in preparing tumor therapy and imaging drug
A technology of cycloquinazolines and cycloquinazolines, applied in the field of tumor treatment and imaging drugs, can solve the problems of high absorption, limited brain tumor imaging applications, false positives, etc., and achieve good anti-tumor activity, Good anticancer activity, less synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028]
[0029] The compound is N-[3-(2-fluoroethoxy)phenyl-(6,7-benzo-9-crown-3)]quinazolin-4-amine, and its synthetic route:
[0030] 1.1 Synthesis of diethylene glycol di-p-toluenesulfonate
[0031] Add 10mL (0.105mol) of diethylene glycol, 300ml of dichloromethane, 40g (0.21mol) of p-toluenesulfonyl chloride into a 500ml round-bottomed flask, and slowly add 47g (0.84mol) of hydroxide Potassium, the reaction is basically completed in 1.5h. The reaction solution was washed three times with water (3×100ml), and the organic layer was collected and dried over anhydrous sodium sulfate. Filtration and spin-drying of the solvent gave the crude product ethanol recrystallization to obtain 40 g of a white solid with a yield of 95%. The obtained spectrum is consistent with that reported in the literature.
[0032]
[0033] 1.23, Synthesis of ethyl 4-(benzo-9-crown-3)benzoate:
[0034] In a 250ml round bottom flask, add 6.68g (48.31mmol) of potassium carbonate, 50ml of DMF, h...
Embodiment 2
[0055]
[0056] The compound is N-[3-(2-fluoroethoxy)phenyl-(6,7-benzo-12-crown-4)]quinazolin-4-amine, its synthetic route:
[0057] 2.1 Synthesis of triethylene glycol di-p-toluenesulfonate
[0058] Add 10mL (0.075mol) of triethylene glycol, 300ml of dichloromethane, 28.6g (0.15mol) of p-toluenesulfonyl chloride into a 500ml round bottom flask, and slowly add 33.6g (0.6mol) of Potassium hydroxide, the reaction was basically completed in 8 hours. The reaction solution was washed three times with water (3×100ml), and the organic layer was collected and dried over anhydrous sodium sulfate. Filtration and spin-drying of the solvent gave the crude product methanol recrystallization to obtain 30 g of a white solid with a yield of 90%. The obtained spectrum is consistent with that reported in the literature.
[0059]
[0060] 2.23, Synthesis of ethyl 4-(benzo-12-crown-4)benzoate:
[0061] In a 250ml round bottom flask, add 10.02g (72.5mmol) of potassium carbonate and 50ml ...
Embodiment 3
[0082]
[0083] The compound is N-[3-(2-fluoroethoxy)phenyl-(6,7-benzo-15-crown-5)]quinazolin-4-amine, its synthetic route:
[0084] 3.1 Synthesis of tetraethylene glycol di-p-toluenesulfonate
[0085] Put 10mL (0.068mol) of tetraethylene glycol, 300ml of dichloromethane, 25.8g (0.136mol) of p-toluenesulfonyl chloride into a 500ml round bottom flask, and slowly add 30.4g (0.544mol) ) Potassium hydroxide, the reaction is basically completed in 1.5h. The reaction solution was washed three times with water (3×100ml), and the organic layer was collected and dried over anhydrous sodium sulfate. Filter and spin dry the solvent to obtain the crude product as an oil. Column chromatography (petroleum ether: ethyl acetate = 1:4) gave 34 g of light yellow oily liquid. The yield was 98%. The obtained spectrum is consistent with that reported in the literature.
[0086]
[0087] 3.23, Synthesis of ethyl 4-(benzo-15-crown-5)benzoate:
[0088] In a 250ml round bottom flask, add 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com