Methacrylate fluorescent monomer with thiazolepyridine structure and preparation method thereof
A methacrylate and thiazopyridone-containing technology, which is applied in the field of fluorescent compound synthesis, can solve problems such as the difficulty in synthesizing fluorescent polymers with a high degree of polymerization, the quantum yield being easily affected by solvents, and the free radical polymerization process. Achieve the effect of mass production, high quantum yield, and small steric hindrance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
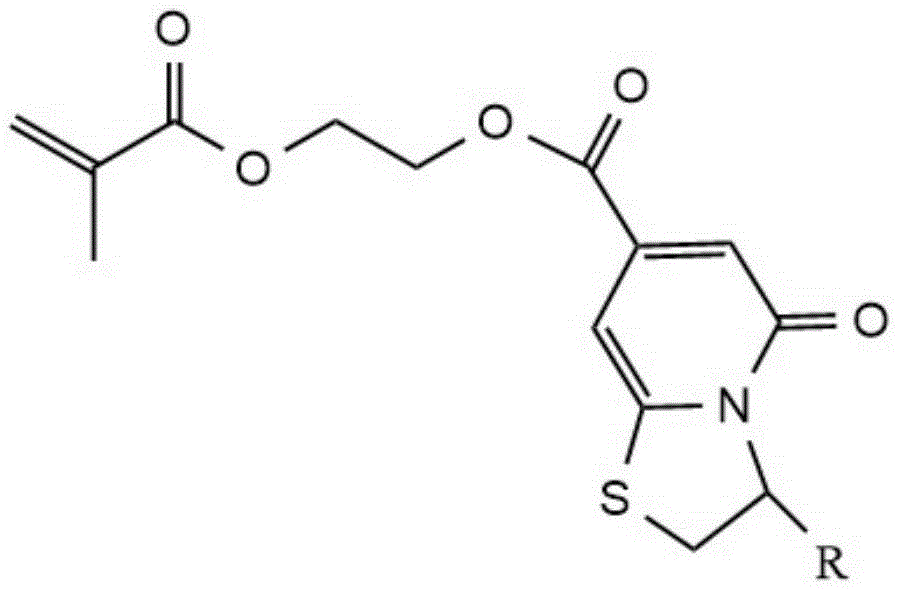
[0028] A preparation method of a methacrylate fluorescent monomer containing a thiazopyridone structure is as follows: weigh 1.95g thiazopyridone-3-carboxylic acid, 2.00mL hydroxyethyl methacrylate, 3.82g 1-ethyl-(3 -Dimethylaminopropyl) carbodiimide hydrochloride, 2.70g of 1-hydroxybenzotriazole and 6.50mL of triethylamine were dissolved in 60mL of N,N-dimethylformamide, under nitrogen protection conditions Stir the reaction at room temperature 20°C for 48 hours, then filter the mixture under reduced pressure after the reaction, add an equal volume of deionized water to the obtained filtrate and dilute with ethyl acetate three times, take the upper organic phase and use the mass fraction Repeatedly washing with 10% citric acid aqueous solution and saturated sodium bicarbonate solution, then drying and rotating the solvent to obtain a crystal methacrylate fluorescent monomer containing a thiazopyridone structure.
Embodiment 2
[0030] A preparation method of a methacrylate fluorescent monomer containing a thiazopyridone structure is as follows: weigh 4.00g thiazopyridone-3-carboxylic acid, 4.00mL hydroxyethyl methacrylate, 8.00g1-ethyl-(3 -Dimethylaminopropyl) carbodiimide hydrochloride, 5.50g of 1-hydroxybenzotriazole and 13.00mL of triethylamine were dissolved in 150mL of dimethyl sulfoxide, under nitrogen protection conditions at room temperature for 20 The reaction was stirred at °C for 24 hours. Then the mixture after the reaction was filtered under reduced pressure, added a sufficient amount of deionized water to the resulting filtrate to dilute and extracted three times with dichloromethane, and the upper organic phase was repeated with 10% hydrochloric acid and saturated sodium bicarbonate solution After washing, drying and rotary evaporation of the solvent, a crystal methacrylate fluorescent monomer containing a thiazopyridone structure is obtained.
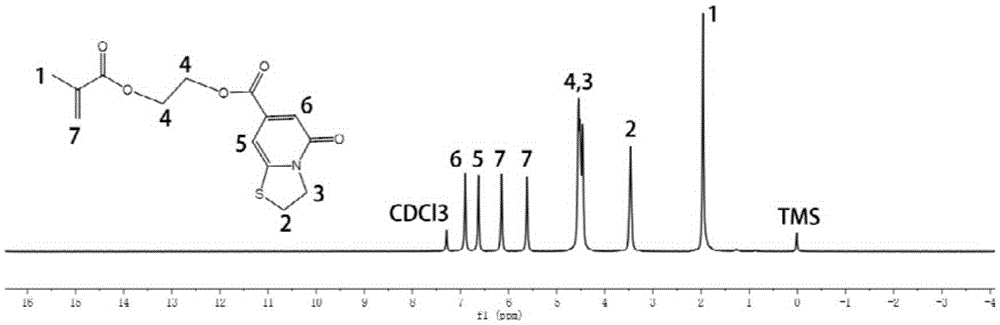
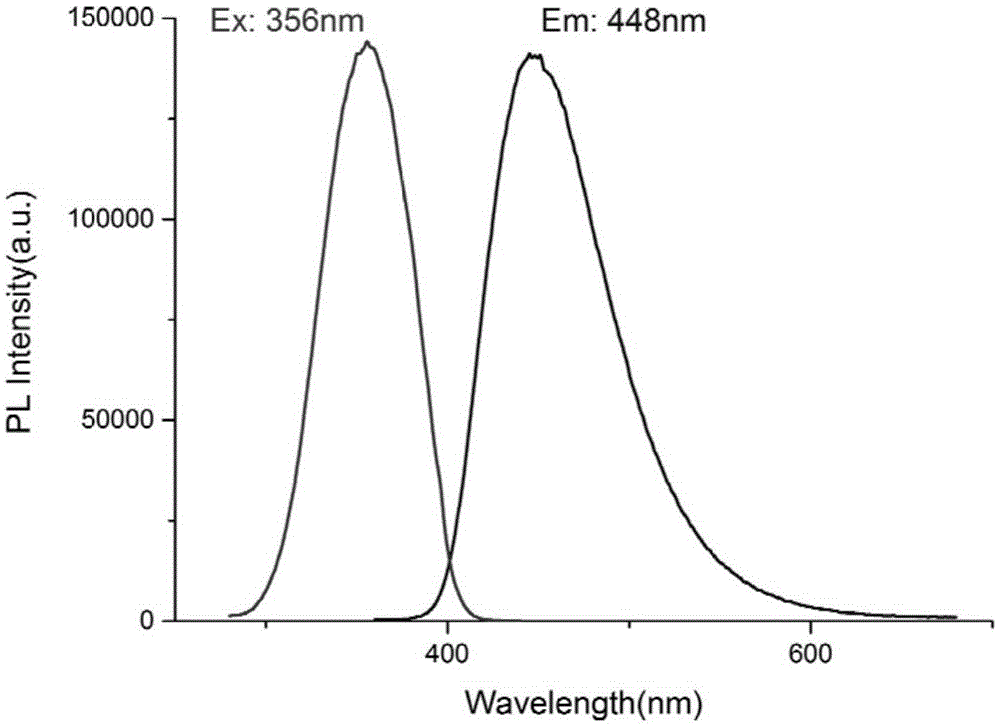
[0031] see figure 1 , the structure of...
Embodiment 3
[0033] A preparation method of a methacrylate fluorescent monomer containing a thiazopyridone structure is: under stirring, thiazopyridone-3-carboxylic acid, methacrylic acid hydroxyl Ethyl ester, 1-ethyl-(3-dimethylaminopropyl) carbodiimide hydrochloride, 1-hydroxybenzotriazole and triethylamine were dissolved in water, and then heated at 40°C under nitrogen protection After reacting for 24 hours, the reaction product was purified. The specific process of purification was as follows: filter the mixture after the reaction under reduced pressure, add deionized water equal to the volume of the filtrate to dilute the resulting filtrate, and extract the polysaccharide with ethyl acetate. Once, the upper organic phase was repeatedly washed with 1% citric acid aqueous solution and saturated sodium bicarbonate solution, then dried and the solvent was rotated to obtain a methacrylate fluorescent monomer containing a thiazopyridone structure. Wherein, the mass ratio of water to thiazop...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com